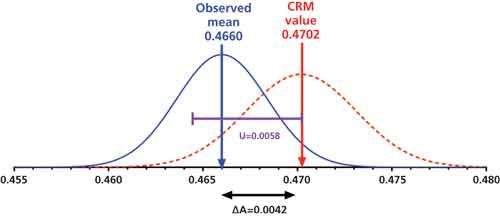
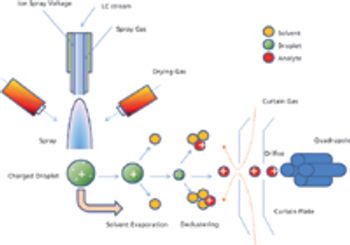
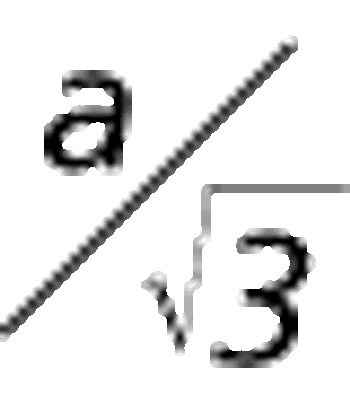As the pharmaceutical industry prepares for changes to compendial and regulatory standards for elemental impurity analysis, QA/QC and laboratory scientists are tasked with adapting their operations to include data management, analysis, and reporting based on inductively coupled plasma–mass spectrometry (ICP–MS).