
Greater sophistication in 3D X-ray imaging technology raises its utility for QA/QC in manufacturing.

Greater sophistication in 3D X-ray imaging technology raises its utility for QA/QC in manufacturing.

Agilent Technologies Inc. introduced the MassHunter BioConfirm application, which allows users to rapidly create targeted screening methods.

Bruker Chemical and Applied Markets offers three LC-triple quadrupole (LC-TQ) mass spectrometers, the EVOQ Qube, the EVOQ Elite, and the EVOQ Elite ER.

Thermo Fisher Scientific Inc. introduced the Thermo Scientific Orbitrap Fusion Tribrid liquid chromatography-mass spectrometry (LC-MS) system, which combines three mass analyzers.

Click chemistry has become a powerful tool in drug discovery, chemical biology, and proteomic applications.
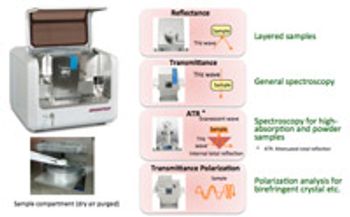
Now at a practical size and price, terahertz spectroscopy is advancing as a nondestructive method of analysis for solid forms and tablet coatings.
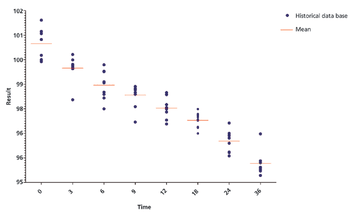
The authors discuss three methods for identification of out-of-trend (OOT) results and further compare the z-score method and the tolerance interval in OOT analysis for stability studies.

Microwave spectroscopy overcomes some of the limitations of circular dichroism and vibrational circular dichroism in analyzing enantiomers.

Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.

Compositing samples is appropriate under certain circumstances but raises caveats on how and when it should be applied.

The author examines dry dispersion and outlines the related analytical method development.

New capillary-based imaging techniques for protein charge variant analysis are reducing development and start-up times for protein-based therapeutics.

Increasing production efficiency while ensuring high parenteral product quality is the goal of both manual and automated visual inspection systems.

Shimadzu's LCMS-8040 combines newly improved ion optics and collision cell technology with proprietary ultrafast technologies.

The process-driven system reduces total cost of ownership.

Iterative and multiple incremental advances in particle-characterization technology are making a difference in pharmaceutical analysis applications.
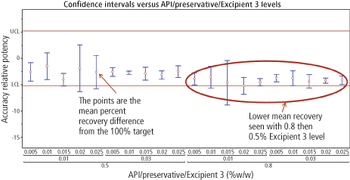
The authors describe a method-validation-by-design (MVbD) approach to validate a method over a range of formulations using both design-of-experiment and quality-by-design principles to define a design space that allows for formulation changes without revalidation.
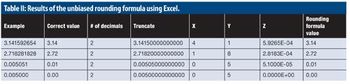
The mysteries of rounding are exposed; strict, unbiased rounding can be applied.

Bristol-Myers Squibb embarks on a multi-year journey to overcome the challenges of serialization and reap the benefits.

Thermo Fisher Scientific Inc. launched new software, instruments, consumables, and services for analytical applications at Pittcon 2013.

Shimadzu Scientific Instruments introduced several new products at Pittcon 2013.

At Pittcon 2013, Water Corp. announced improvement to chromatography systems and software.

Ultra high-pressure liquid chromatography (UHPLC) is enabling faster product development and production batch approvals with increased sensitivity.

Instrument manufacturers and testing service providers are offering improved tools to biologic charaterization.
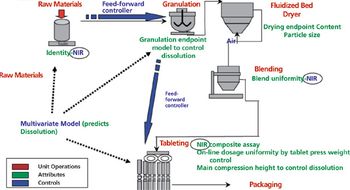
The authors present topics discussed and conclusions that resulted from the PDA QbD workshop.