
Effective May 1, 2010, the US Pharmacopeia has revised its Pending Monographs Guideline to clarify that excipients are eligible for pending-monograph status and can ultimately be included in an official National Formulary (NF) monograph.

Effective May 1, 2010, the US Pharmacopeia has revised its Pending Monographs Guideline to clarify that excipients are eligible for pending-monograph status and can ultimately be included in an official National Formulary (NF) monograph.

Chemocatalysis and biocatalysis are important elements of an effective strategy for improving yield and stereoselectivty.

Agreement on standards for excipient qualification, development, and fair pricing is underway.

A Technical Forum featuring representatives from Dow Chemical, ISP, and DMV-Fonterra Excipients. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The author describes the new IPEA excipient good-manufacturing-practice certification program that is now ANSI accredited. This article is part of a special issue on excipients and solid dosage.

The authors review new regulatory expectations and describe potential approaches to accommodate excipient variability. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The author proposes techniques, based on Six Sigma methods, for monitoring such processes to discover their airflow patterns and reduce opportunities for spillage.This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

A Q&A with the First Federation Chair, Patricia Rafidison.

The authors describe the origins of single-use components and explain their application to aseptic processes. They also show how disposable devices have changed over time and offer a glimpse of the future.

Growth in the market for monoclonal antibodies, recombinant proteins, and vaccines creates new opportunities for drug companies and suppliers.

Emerging markets remain an important element in the strategies of pharmaceutical companies and their suppliers.

It's time to maintain a thorough traceable excipient trail.
The second annual Pharmaceutical Technology Bioprocessing Survey offers a snapshot of the industry following 2009's megamergers.

Pharmaceutical Technology talked to drugmakers, equipment vendors, and service providers to learn more about the advantages and disadvantages of rapid microbial-detection methods.

As contract manufacturers and drug companies meet at Informex, the stage is set for the latest in pharmaceutical chemical development.

Leading experts share insight on the current and future direction of process analytical technology. This article contains bonus online material.
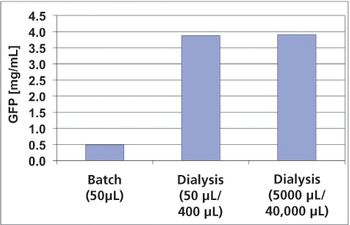
The authors sought to improve the productivity of protein synthesis by using a highly active cell-free extract from Escherichia coli and by optimizing buffer conditions and shaking conditions.
Leading experts share insight on polymorphism and crystallization.

The authors discuss a study that demonstrates the use of polyethylene oxide mixtures to investigate the effect of polymer viscosity on formulation robustness.

In response to a request from the FDA, the US Pharmacopeial Convention (USP) has revised its standards for propylene glycol and sorbitol solution, excipients widely used in prescription and over-the-counter drugs.

In response to a request from the US Food and Drug Administration, the US Pharmacopeial Convention (USP) revised its standards for propylene glycol and sorbitol solution

Functionalized supramolecular catalysts and an enantioselective route to unnatural amino acids are some recent developments.

A recent book reminds readers that small-molecule chemistry has enabled advances in biotechnology.
The authors explain a process for moisture-activated dry granulation in detail and provide guidance for the selection of excipients and equipment.
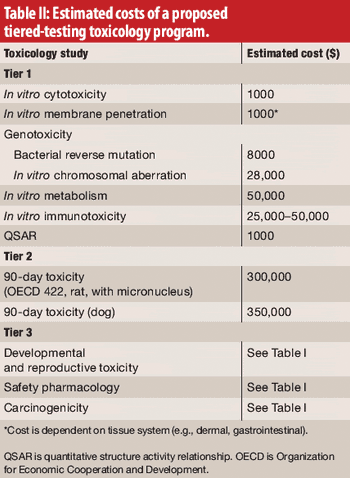
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.