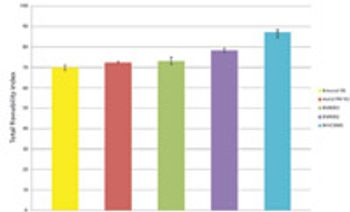
The authors investigated the tableting properties of PanExcea MHC300G, a high-performance excipient.

The authors investigated the tableting properties of PanExcea MHC300G, a high-performance excipient.
A new audit guide aims to improve supply-chain security and supplier qualification practice.

The power of emerging markets is reflected in the pharma's sales and production positions.
Will 2011 be a more promising year for new molecular entities? A review of Big Pharma's late-stage pipeline shows what might lie ahead.

Improvements in analytical techniques may call for a re-evaluation of the biopharmaceutical industry saying that "the process is the product."

During the past few years, we've seen a growing interest in direct oral application products, evidenced by the large amount of products already available on the market.

Iain Moore explains what progress has been made so far regarding EU plans to regulate excipients.

Biologics are large molecular weight molecules primarily formulated for parenteral administration; however, there are some smaller biomolecules that have been formulated for oral use.

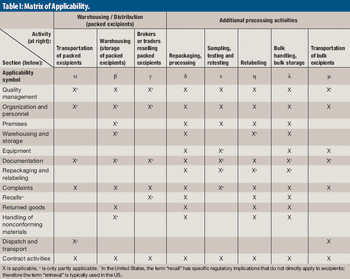
Excipients should be regulated to manage risk. Although, excipients are an integral part of a finished pharmaceutical product, the issue of regulation is complex.
Contract manufacturers strengthen their toolboxes and partnerships as they navigate the changing drug-development model.

Excipient purity is critical in all applications of drug formulation. Typically, the more sensitive or reactive an API, the more critical excipient purity becomes.
Approaches in cyclization, palladium-catalyzed cross couplings, fluorination, and natural product synthesis help to optimize routes for select drugs.

IPEC extends its reach to Brazil and Argentina in an effort to harmonize excipient best practices.

Abuse-deterrent combination drugs represent a niche area in formulation development.

Industrial and academic partnerships forge new territory in solid-state chemistry.

The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.

Stuart Needleman, president of active-ingredient development and manufacturing at Aptuit, discusses industry trends and challenges.

A recently released industry guide outlines a science- and risk-based approach to control the risk of cross-contamination.
Drugmakers hatch new manufacturing paradigms in the wake of the 2009 H1N1 influenza pandemic.
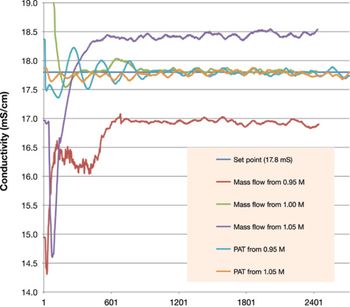
The authors describe the operational requirements and design of a process-ready PAT-based IBD system.

The author describes the development of small-angle X-ray scattering and analyzes its advantages in the characterization of drug-delivery systems and large molecules. This article is part of a special Analytical Technology issue.

US Pharmacopeia apparatuses for testing the dissolution of transdermal drugs produce good, reproducible results. Yet some scientists believe that further modifications could improve the instruments? suitability for this application.

Pharmaceutical Technology gains insight into approaches for producing aromatic amines.
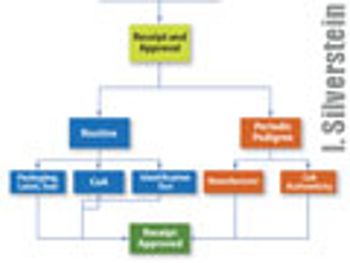
The author explains the background behind the excipient pedigree and how to implement its use.

FDA chemistry reviewers in the Office of Generic Drugs provide an overview of common deficiences cited throughout the Chemistry, Manufacturing, and Controls section of ANDAs.