
Industry and academia advance novel approaches for achieving enanioselectivity.

Industry and academia advance novel approaches for achieving enanioselectivity.

USP optimizes identification tests and impurities procedures.

A Q&A with BASF moderated by Patricia Van Arnum.
The authors explain chemical transformations that are achievable through certain biocatalytic routes.

Approaches to scaling up API syntheses center on ways to optimize process conditions and operability.
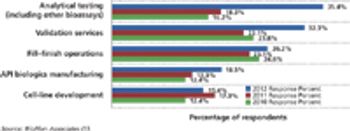
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

A complete and unedited audio slideshow of the Excipient Fest roundtable on atypical visible particles

Flow chemistry and microreactors offer an alternative to traditional batch manufacturing.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

Flow chemistry and microreactors offer alternatives to traditional batch manufacturing.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2012 edition from EMD Millipore and Meissner Filtration Products.

Peptides and related technologies to are starting to improve production.
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.
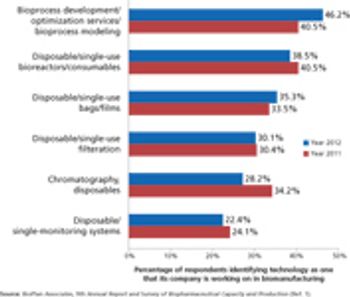
Industry wants more innovation, but can suppliers meet customer needs?

Targeted polymeric nanoparticles are an important vehicle for controlling and targeting dosing of chemotherapeutic agents.

Advances in palladium-catalyzed hydrogenation, visible-light photocatalysis, and chemocatalyisis for heterocycles are some recent developments.
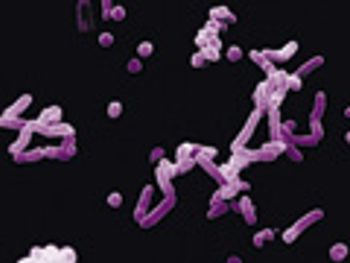
In this technical forum, experts describe different methods of rapid microbial testing and their applications.

Contract API manufacturers and fine-chemical producers roll out capacity and service expansions.

Excipient manufacturers expand production capacity and partner to broaden their offerings.

On Mar. 7, 2012, GE Healthcare announced an agreement to acquire Xcellerex, a supplier of manufacturing technologies for the biopharmaceutical industry, for an undisclosed amount.

Regulatory bodies, standard-setting organizations, and industry seek to tackle the problem of counterfeit drugs and securing the flow of pharma ingredients.

The author reviews significant changes to GMP for excipients in the forthcoming American National Standard, including a risk-based approach to excipient manufacture, why new requirements were proposed, and their potential impact to excipient manufacturers.

This article provides guidance for industry on how to comply with the pending American National Standard on excipient GMP, with a focus on risk assessment.

As biopharmaceutical development and commercialization increases, companies are expanding their cold-chain capabilities.