
Text from a merger prospectus reveals the termination fee for the proposed Pfizer-Allergan deal would change if any new tax regulations were passed prior to the closing of the transaction.

Text from a merger prospectus reveals the termination fee for the proposed Pfizer-Allergan deal would change if any new tax regulations were passed prior to the closing of the transaction.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Integrated pharmaceutical blister-packaging equipment systems strengthen serialization and brand protection capabilities.
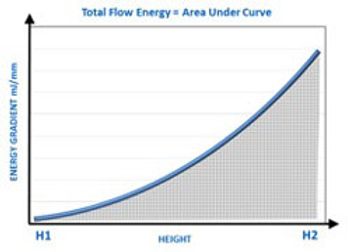
Dynamic powder testing and measurement of bulk powder properties can complement shear cell testing to identify the causes of poor hopper performance in solid-dosage drug manufacturing.

Experts at the ISPE annual meeting describe best practices, including containment and production in classified spaces.

BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.

The UK’s National Biologics Manufacturing Centre will use Novasep’s BioSC Lab for protein purification.

AstraZeneca’s solid-dose facility in Taizhou, China received ISPE’s FOYA 2015 Overall Winner award.

Even though rising production and use of generic pharmaceuticals is saving billions for the nation’s healthcare system, policy makers continue to slap the industry with policies it claims will limit product development and sales.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

Biotech boom, niche markets, smaller batch sizes and high potency manufacturing are among the key trends shaping the pharmaceutical industry of the 21st century, according to Christian Treitel from Bosch Packaging Technology.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.

At least 70 patents for Humira will protect the legacy product from biosimilar competitors, according to information presented during the company’s third-quarter earnings call.
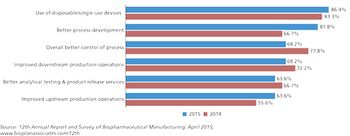
Better process development is creating industry benchmarks for bioprocessing.
Delamination of glass packaging is a source of particulates in parenteral drugs, but identifying the root cause allowed the design of an improved manufacturing process for glass vials.

Industry experts share insights on the advances in tablet coating technologies and the potential of continuous coating in solid-dosage manufacturing.

Sartorius Stedim Biotech’s Flexsafe 3D Pre-designed Solutions meet ASTM D4169 Standard Practice requirements and are designed for storage and shipping of biopharmaceutical fluids.

Although shortages, quality, and regulatory challenges remain, improved technologies and new investments suggest that the worst may be over.

Miriam Beyer, European marketing manager, West Pharmaceutical Services, describes causes of recent parenteral drug shortages.

ProBioGen plans to develop and scale-up production of a biosimilar to the mAb cancer drug trastuzumab and will transfer the commercial manufacturing process to Indonesia’s government-owned Bio Farma.

USP responds to FDA's draft guidance on the naming of biological products.

Catalent’s SMARTag™ technology enables biologic innovators to develop more efficacious antibody drug conjugates. It allows site-specific, programmable drug placement using proprietary cytotoxin-linkers/conjugation chemistry in an efficient and scalable process.

Capsugel has launched its enTRinsic drug-delivery technology platform. Using pharmaceutically approved enteric polymers, the technology offers full enteric protection without the need for functional coatings and enables targeted release of gastric acid- and heat-sensitive active ingredients in the upper gastrointestinal tract. The new technology platform expands Capsugel’s range of modified- and targeted-release solutions and biotherapeutic formulation offering, and can be used for oral delivery of drugs, including vaccines, proteins, and peptides.

According to a third-quarter earnings report, Pfizer’s vaccine business contributed nearly 14% to its total revenue.