
In our tablet coating process, we are losing up to 15% of our coating solution in each processing run. What can we do to prevent this problem in order to reduce waste and increase our cost efficiency?

In our tablet coating process, we are losing up to 15% of our coating solution in each processing run. What can we do to prevent this problem in order to reduce waste and increase our cost efficiency?

On Mar. 7, 2012, GE Healthcare announced an agreement to acquire Xcellerex, a supplier of manufacturing technologies for the biopharmaceutical industry, for an undisclosed amount.

AstraZeneca Files Suit Against FDA; Pfizer, Biocon Terminate Biosimilar Alliance; and More.

CML - Solid State Chemistry

BASi Restructures; Celerion, Ricerca Biosciences Form Biosimilars Alliance; and More.

The Bulk Pharmaceuticals Task Force, an affiliate of the Society of Chemical Manufacturers and Affiliates (SOCMA), issued its support for the introduction of a House bill, the Generic Drug and Biosimilar User Fee Act (HR 3988), which SOCMA says will help to achieve parity between foreign and domestic firms.

GlaxoSmithKline and Daiichi Sankyo have formed a joint venture that they claim will create the biggest vaccines company in Japan. The joint venture will seek to improve access to vaccinations in the Asian nation as well as introduce new vaccines.

The divide between innovation and conflict of interest in medical research is not so clear.

Has the long-awaited guidance answered all of the industry's questions?

The article examines some recent developments for this process step and for continuous manufacturing overall.
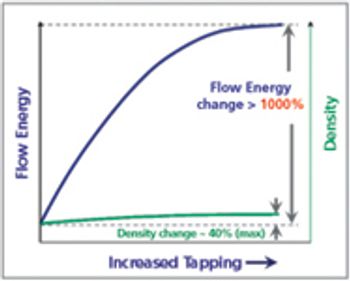
This article considers the different conditions to which the powder is subjected in the tableting process, and discusses which powder properties should be measured to accurately reflect likely powder behavior in the process.

Taste-masking is an important consideration to ensure patient compliance.

Experts in solid dosage discuss the formulation and manufacture of multilayer tablets.

FDA answers key questions about the October 2011 guidance on using physical–chemical identifiers in solid oral dosage products to help prevent and avoid counterfeiting.

The pharma industry has reached the long-dreaded patent cliff, but for copycat products, business is booming.

JHS Secures Four Sterile Parenteral Products Manufacturing Contracts; Samsung Biologics, Biogen Idec Establish Biosimilars Joint Venture; and More.

Pfizer has signed an agreement with the Chinese biopharmaceutical company Zhejiang Hisun Pharmaceuticals with the objective of establishing a $545-million joint venture to develop and commercialize branded generic medicines in both China and the global market.

PDA organizes this conference to address the technical and regulatory issues related to parenteral packaging. Chemical, physical and microbiological aspects will be considered and current regulatory challenges will be discussed. Two Training Courses on Container Closure Development or Selection and Utilization of Glass Containers in Pharmaceutical Packaging will be given.
The author discusses the relative advantages and disadvantages of lyophilization in vials and dual-chamber systems.

A nickel's worth of free advice to the competition could come at the expense of your bottom line.

Debottlenecking downstream mAb purification.

Experts discuss solutions for filter bacterial retention and related challenges. Contains online bonus material.

The generic-drugs market is poised to experience strong growth as key blockbuster products go off patent, but companies looking to benefit from this will have to be careful about the product segments where they compete, according to a report from Frost & Sullivan.

The pharmaceutical majors advance vaccines for the developing world.

The authors designed an upper punch with a removable punch tip to determine a tablet formulation's propensity to stick by weighing the mass of powder adhered to the punch tip.