
AstraZeneca Acquires Chinese Generic-Drug Company; Takeda Makes Management Changes; and More.

AstraZeneca Acquires Chinese Generic-Drug Company; Takeda Makes Management Changes; and More.

Pfizer and GlaxoSmithKline have announced separate agreements with the GAVI Alliance to supply pneumococcal vaccines to developing countries.

Last week, the US Department of Health and Human Services and Novartis Vaccines and Diagnostics dedicated a manufacturing plant that can create influenza vaccine using cultured animal cells instead of the conventional expression system of fertilized eggs.

Samsung and Biogen Idec agreed to invest $300 million to establish a joint venture to develop, manufacture, and market biosimilars. The deal is Samsung's latest effort to strengthen its position in biosimilars.

On Dec. 6, 2011, FDA announced that a public meeting will be held on Dec. 16, 2011 to discuss recommendations for a user fee program for biosimilar biological products for fiscal years 2013–2017.
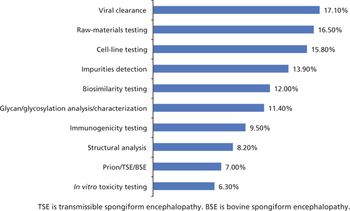
Biosimilar manufacturers need better expression systems and analytical tools to compete.

Pharma companies must balance demand for new drugs while facing reduced R&D spending.

Contamination is almost always related to human error and there is a clear drive to reduce human implications in aseptic operations. This can be achieved in multiple ways.

On Sept. 27, 2011, FDA sent Genentech a Form 483 listing several violations at the company's South San Francisco, California, plant. The violations included problems with investigations into batch failures, inappropriate equipment design, and insufficient protection against contamination. FDA visited the plant, which produces the cancer drug Avastin, 13 times in September 2011 and made four observations.

Small drug companies with hopes of achieving $1 billion in sales can pursue various strategies.

Biosimilar manufacturers need better expression systems and analytical tools to compete.

The authors discuss the analysis of the resulting data, focusing on methods for the calculation of mass median aerodynamic diameter, one of the metrics routinely used for comparative testing.

The future, and increasingly the present, for aseptic operations is isolated robotics. Companies that wish to gain competitive advantages in their operations are stepping up and taking notice.

EMA released two concept papers for consultation that address the need to revise existing guidelines on biosimilar medicines and influenza vaccines.

Average deal size and amounts invested increased in the second quarter, but totals trail funding of last year.

Manufacturers fund research and reduce prices to tackle diseases.

This risk-management case study focuses on assessing empty capsules.

In a 2004 guidance, FDA says that the "use of redundant sterilizing filters should be considered in many cases." But not all manufacturers agree on what redundant filtration is.

The International Society for Pharmaceutical Engineering will soon publish an update for its guide to sterile-product manufacturing facilities. The new publication will replace the original guide and contain practical information about technological advances in sterile manufacturing.

Meeting Co-Chair Susan Schniepp provides insight into this year's regulatory conference.

Representatives from FDA, the Generic Pharmaceutical Association, the European Fine Chemicals Group, and a task force from the Society of Chemical Manufacturers and Affiliates are nearing completion of draft legislation for the Generic Drug User Fee Act.

Powder Systems Ltd's (PSL's) new GFD™ isolator combines the latest innovation to provide a high containment lab scale filtration and drying solution with the same reliability and benefits as PSL high containment production size filter dryers.
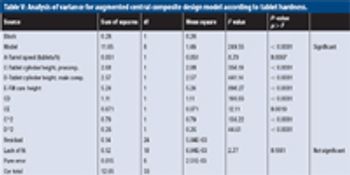
The author prepared and analyzed a detailed design of experiments for the manufacture of a simple tablet formulation. The aim was to test whether tablet hardness and weight could be controlled during the compression process by adjusting certain machine parameters.

Elham Blouet from Roquette explains the importance of carbohydrates for injection and the challenges in this niche market.

A Q&A with Joe Cascone, director of potent pharmaceutical development at Metrics, moderated by Patricia Van Arnum. Discussion of the key considerations made in facility design, equipment selection, and operations to achieve desired levels of containment.