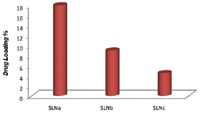
The main challenge for tablet manufacturers processing highly potent APIs (HPAPIs) is to protect equipment operators from the inhalation of airborne particles and prevent skin contact with the product during the entire production process: dispensing, granulation, tablet compression, coating and packaging.