
Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...

Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...

The National Nanotechnology Initiative's new strategic plan affirms the federal government's support for research and development in nanotechnology, including for medical and healthcare applications.

Merck Sells Facility to Cherokee Pharmaceuticals, Inflazyme Announces Senior Management Resignations, More...

There is a need for current cleaning validation methods to be used for topical formulations. The authors highlight the issues and challenges encountered.
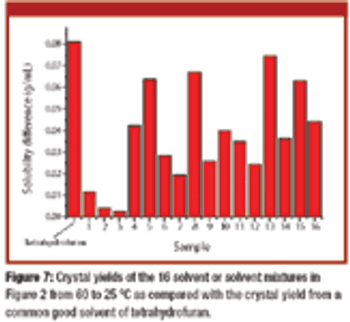
The authors propose extending initial solvent screening for a single-solvent system to the cocktail solvent screening of binary and ternary solvent mixtures.

FDA’s May 1999 container-closure guidance has accelerated the requirements for extractable and leachable testing of container-closure packaging components. Since that date, additional recommendations have been made by industry groups to clarify how these issues should be addressed.
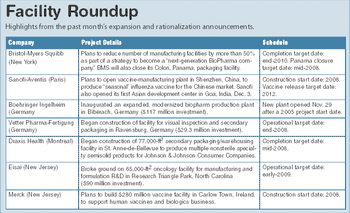
News, world briefs, facility roundup, events and other items for January 2008.
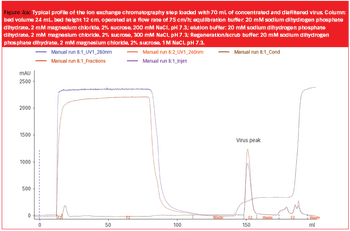
The use of viral vectors as gene delivery and vaccine vehicles has developed rapidly during the last two decades owing to several viral properties. Viruses can infect cells efficiently, often have a broad tissue tropism and can achieve very high levels of either stable or transient transgene expression. Furthermore, their intrinsic immune-stimulatory properties can have adjuvant effects during the treatment of cancer or infectious disease and, importantly for manufacturing scale-up, some viruses can be grown to very high titre (.1012 particles/mL). The development of robust production procedures is essential to move therapeutics that utilize viral vectors into clinical trials, and to make them cost effective for market supply. Here, we describe some of the aspects of production that must be considered and optimized when producing virus vectors on an intermediate or large scale. By drawing examples from our experience of vector production, we show that upstream and downstream processes must be designed..

Millipore and Novozymes Form Alliance, Applied Biosystems Names President and CEO, More

Merck & Co. initiated a voluntary recall of 11 lots of its Haemophilus influenzae type B vaccine, Pedvaxhib, and two lots of its combination Haemophilus influenzae type B/ hepatitis B vaccine, Comvax.

Company and People Notes: West to reduce workforce, Eli Lilly's CEO and chairman to retire, more...

Tomorrow, Dec. 14, the US Food and Drug Administration?s Nonprescription Drugs Advisory Committee will discuss the safety and effectiveness of over-the-counter (OTC) cold medications containing phenylephrine.

Company and People Notes: Eisai Acquires MGI Pharma, Ceregene Names CEO, More

Researchers at the University of Kentucky (KY, USA) have located a gene that kills cancer cells while leaving normal cells intact.

Company and People Notes: Boehringer Ingelheim Expands Facility, Regulus Therapeutics Names President and CEO, More
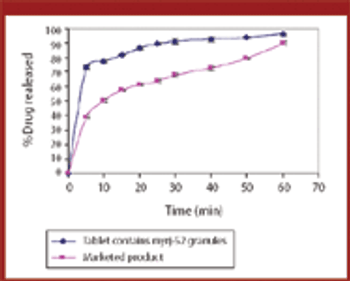
Various manufacturing techniques can improve a drug's solubility, thus increasing its bioavailability. The authors examined whether melt granulation can enhance drug solubility using meloxicam as the drug substance and myrj-52 as the binder.

To keep up with applications, FDA promotes new testing and administrative methods.

In honor of Pharmaceutical Technology's 30th anniversary, the editors conducted a survey of 320 readers last spring to discuss industry advances and future directions. Here are some of your foward-looking responses.

Individualized dosing for specific patient needs has been the goal of medical and pharmacotherapy specialists since they first envisioned pharmacogenetic evaluation. With the measurement of individual levels of metabolism, the optimum dose can be calculated for each individual patient.

Nanotechnology offers an unprecedented opportunity in the rational delivery of drugs and vaccines (1, 2–4).
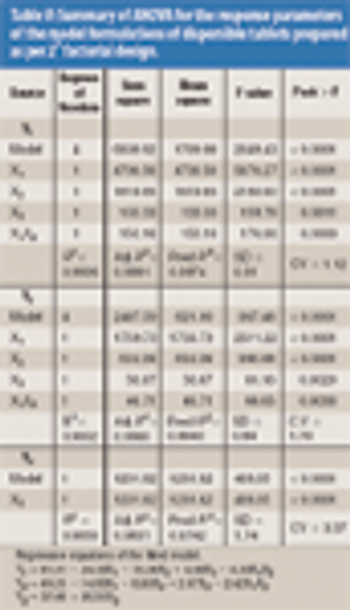
The authors analyzed the effects of complexation as well as the levels of ammonium bicarbonate and crospovidone on tablet wetting time (WT), disintegration time (DT), and percent dissolution efficiency at 60 min (%DE60).

"May you live in interesting times," goes the allegedly ancient Chinese curse. Well, these have been "interesting times" indeed for those working in the pharmaceutical industry.
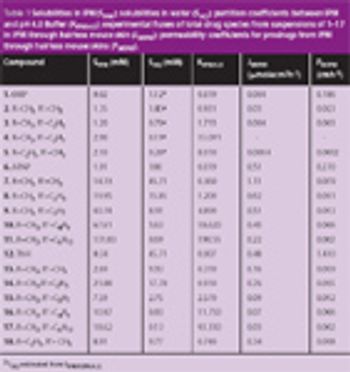
The permeation of drugs through the skin is compromised by the presence of polar functional groups such as thiols, alcohols, phenols, imides or amides. By transiently masking these polar functional groups as prodrugs the permeability of drugs containing these functional groups through the skin can be improved.

Incorporating quality and economy into downstream purification processes can expedite first-in-human clinical trials, product licensure and technology transfer. Experience in the chromatographic purification of biopharmaceuticals enables the use of downstream processing heuristics to produce target molecules in cost-effective processes suitable for regulatory scrutiny.
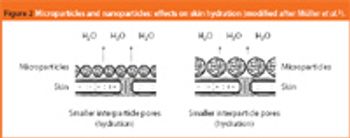
Lipid-based drug delivery systems - such as liposomes, micro-and nanoemulsions, self-emulsified drug delivery systems, and solid lipid micro-and nanoparticles - are becoming more popular because lipid materials are easily characterized, contain a high range of well-defined/tolerated surfactant molecules and can be developed for several administration routes.