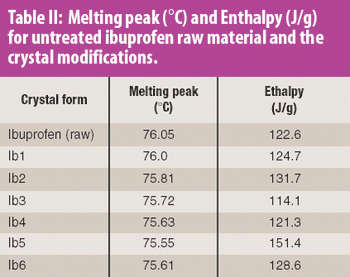
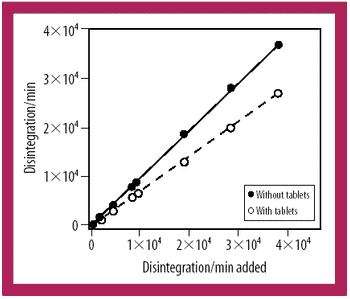
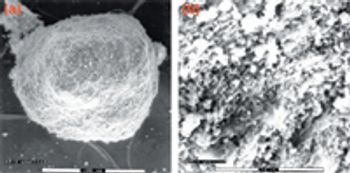
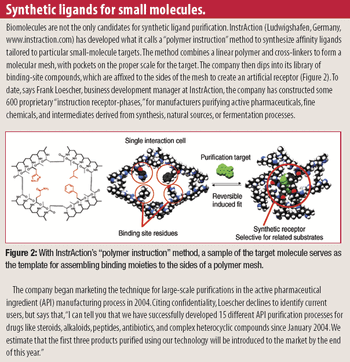
When it comes to developing a robust lyophilization process, formulators can "pay now or pay later," says Jeff Schwegman, PhD, founder and chief scientific officer for BioConvergence. Because 30% of new drugs in clinical trials are biotech-based therapeutics (compared with 7% 10 years ago), more than ever, the US Food and Drug Administration is paying close attention to lyophilization data and questioning pharmaceutical companies about their development cycles, especially cycle development transfer, shelf-temperature mapping, dryer-to-dryer comparison studies, formulation time, process validation, and cycle deviation. Consequently, this is pushing formulators to optimize formulation variables, conduct additional testing during early-stage development, and understanding critical process parameters, equipment qualifications, and manufacturing conditions that can influence formulation behavior at a large scale. Not taking the time or effort to achieve these goals during early development could lead to redundancies in formulation work - a reality observed too often in today's practices.