
The author points out some major obstacles to effective scale-up and describes methods available to pharmaceutical scientists for addressing scalability issues.

The author points out some major obstacles to effective scale-up and describes methods available to pharmaceutical scientists for addressing scalability issues.

In the manufacture of many pharmaceutical products, dry-particle blending is a critical step that directly affects content uniformity.

Novartis to Expand Generics Hold

CBER Draft Guidance for Spore-Formers and Plasmid Vaccines

Generic Drug for HIV/AIDS Gains FDA's Tentative Approval

Extrusion-spheronization and pellet compression are effective means of developing first-order kinetic, controlled-release drug delivery systems of azithromycin (AZI). The authors prepared, evaluated, and optimized AZI formulations and assessed the stability of the selected formulation under accelerated storage conditions.

The Impurity Profiling Group reviews stress testing according to regulatory guidance documents. The authors emphasize what should be considered for late clinical phases and for registration application dossiers.

ISA-88 Automation Standard Gains IEC PAS Status

Albumin-Bound Nanoparticle Drug Nabs FDA Approval

MHRA to Reinspect Chiron Flu Vaccine Plant

Cellular Chemical Factory Lowers Cost of Malaria Treatment

Engineered Oilbodies Produce Vaccines and Adjuvants

Engineered Oilbodies Produce Vaccines and Adjuvants

The author reviews the effects of moisture on flow properties, tensile strength, Heckel plot, energies involved in compaction, and elastic recovery.

From solving complex formulation challenges to delivering full product-development programs, outsourcing services organizations provide valuable expertise, experience, and technical know-how.

The authors examine the effects of three superdisintegrants on the dissolution and absorption of tenoxicam from solid-dispersion formulations.

This article examines the different types of dessicants avaliable to the pharmaceutical industry. It provides information on choosing the right type, and how and when it should be used.

Microspheres of Eudragit RL were developed for colonic delivery of albendazole. The effects of polymer concentration, stirring rate, and concentration of emulsifier on particle size and drug loading were studied. A comparative in vitro drug release study of the optimized formulation was carried out.

Researchers are improving photodynamic therapies with new photosensitizers and light sources for deeper tissue penetration, more site-specific treatments, and reduced side effects.
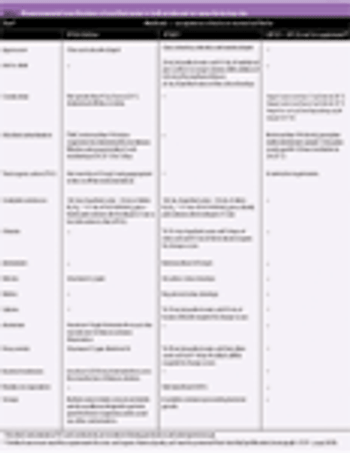
A harmonized global specification is possible providing that the procedures and acceptance criteria defined are acceptable to regulatory authorities in all regions.

Pharmaceutical Science & Technology News

Pharmaceutical Science & Technology Innovations

With creative engineering and sophisticated chemistry, industry scientists offer big solutions for attaining small particle sizes and narrow distributions.

This article describes a novel approach to a scale-up management system, based on a holistic view of the scale-up lifecycle and an accompanying electronic development record of the information created.

Pharmaceutical science and technology news