
The vaccine is a non-live, recombinant subunit vaccine that combines an antigen and an adjuvant system to trigger a targeted and long-lasting immune response to the shingle-causing virus.

The vaccine is a non-live, recombinant subunit vaccine that combines an antigen and an adjuvant system to trigger a targeted and long-lasting immune response to the shingle-causing virus.

The company announced plans to expand its viral vector process development facilities in the United States and its cleanroom facilities in the Netherlands for vector-based products.

Dow’s portfolio of solutions for pharmaceutical applications now includes products from Dow Corning’s silicone technology platform.

Samsung BioLogics’ second facility adds 152,000 L of mAb drug substance capacity.

The committee has voted unanimously to approve Spark Therapeutics’ gene therapy candidate, Luxturna (voretigene neparvovec), for treating a genetically inherited blindness.

West will be showcasing its range of packaging components and drug delivery solutions, including NovaPure components, LyoSeal caps, and the SmartDose platform.

The companies have entered into a strategic collaboration to establish a new cell therapy and regenerative medicine manufacturing platform, which includes a new manufacturing facility in Belgium.

The company is investing EUR 170 million (US$201 million) to expand its vaccine manufacturing site in France for its newest influenza vaccine.

The collaboration will focus on developing a novel and differentiated challenge model for respiratory syncytial virus.

Amgen is seeking approval for an additional indication in glucocorticoid-induced osteoporosis for its blockbuster osteoporosis therapeutic, Prolia.

AstraZeneca is seeking approval for its anti-cancer monoclonal antibody, Imfinzi, for treating non-small cell lung cancer in the European Union.

BARDA has entered a $12-million contract with biopharmaceutical firm Achaogen for the late-stage development of an antibiotic against resistant bacteria, and as a potential treatment for biowarfare agents.

Johnson & Johnson’s Janssen Sciences Ireland UC will invest more than EUR 300 million (US$355 million) to expand manufacturing capacity for biologic medicines at its Ireland facility.

Under an agreement, ProBioGen will develop a stable cell-line for and manufacture an anti-cancer antibody for Chiome using its proprietary cancer cell-killing technology.

Under Project BioShield, the agency could provide more than $170 million in funding to purchase and support late-stage development of Ebola vaccines and therapeutic drugs.

The Danish biotechnology company has been awarded a sole-source BARDA contract valued at more than $539 million for a freeze-dried smallpox vaccine.

Virgin Atlantic Cargo and Delta Cargo have opened a new Pharma Zone at their joint facility at London Heathrow Airport for the handling and storage of pharmaceutical shipments.

A grant from the Bill & Melinda Gates Foundation will advance PnuVax’s pneumonia vaccine’s clinical development and biomanufacturing scale-up using a low-cost manufacturing approach.

CDMOs have established strategies for handling new chemical entities with unknown biological activity.

The pharmaceutical industry is under tremendous pressure to make drug development faster and cheaper. Applying the right formulation strategy using structured and rigorous science can help avoid costly failures and re-starts, but it’s important to start from an early stage.

Applying the right formulation strategies early in the drug development process can help avoid costly late-stage failures.
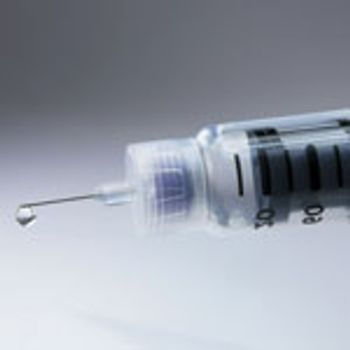
Choosing between presterilized and bulk sterilized prefilled syringes.

Tools aid scale-up and comparison of single-use and stainless-steel bioreactors.

Fluid Air’s PolarDry spray dryers use electrostatic technology, providing low-temperature spray drying, encapsulation, and selective agglomeration in the creation of particles.

Capsules offer certain benefits over tablets for oral-solid dosage drugs, and several types of capsules are available.