
Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

Researchers were not able to replicate all of the findings in five highly-cited cancer biology papers.

In a blog post, Robert Califf and Rita Nalubola discuss the agency’s approach to the use of genome-edited products.

FDA released a long-awaited draft guidance to help sponsors seeking to demonstrate interchangeability for biosimilar products.

An FDA guidance on biosimilar naming garnered mixed responses from the Biosimilars Forum, the American College of Rheumatology, and Pfenex.


The membrane-based Protein A purification tool was unveiled at the 2017 PepTalk Conference in San Diego, California.

Language surrounding regenerative medicine and the REGROW Act appeared back into the 21st Century Cures Act right before it passed. What will this mean for the controversial testing and marketing of stem-cell therapies?

The companies will combine expertise on T-cell therapies with two or more binding domains to create novel oncology medications.

The partnership will focus on providing practical information to clients on the development of biologics and vaccines.

FDA plans to advance initiatives for ensuring reliable production of drugs and biologics in 2017.

Analytical technologies play a key role in the characterization and quantitation of oligonucleotide therapeutics.

A pilot project, beginning in 2017, will support the development of biosimilars.

Univercells received a grant from the Bill & Melinda Gates Foundation for the development of a vaccine manufacturing platform.

Oxford Genetics received £1.61 million from Innovate UK to explore computational and synthetic biology approaches for optimized mammalian bioproduction.

GlaxoSmithKline opened a new vaccines R&D center in Rockville, MD creating up to 200 new jobs.

Researchers test the efficacy of a new polymer that is an alternative to PEG for drugs used to treat type 2 diabetes.
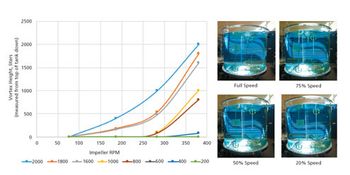
Quantitative and qualitative tools allow better understanding of mixing in a single-use bioprocessing system.
Hot-melt coating was used to develop taste-masked orally disintegrating granules of acetaminophen and caffeine.

Takeda will invest more than 100 million Euros to build a new manufacturing plant for its dengue vaccine candidate in Singen, Germany.

Oxford Genetics has been awarded a grant to develop packaging cell lines for virus bioproduction and will work in collaboration with the University of Oxford to generate cell lines for the scalable manufacture of retrovirus and lentivirus vectors.

Avecia is adding drug substance capacity at its Milford, MA manufacturing site.

Research by agency scientists may help speed development of Zika virus vaccines.

Ensuring that materials have optimal critical quality attributes for the required formulations is crucial.

Idifarma has announced a growth of more than 50% over the past two years. Revenue is expected to hit approximately EUR5.3 million in 2016, growing from EUR3.5 million in 2014.