
Forecasts were lowered, Reuters reported, due to reduced drug approvals and increased competitive pressures.

Forecasts were lowered, Reuters reported, due to reduced drug approvals and increased competitive pressures.

Recent legislation and PDUFA initiatives aim to streamline oversight and testing requirements.
Advances in chemical synthesis are enabling greener, more cost-efficient processes for API manufacturing.
Transdermal and inhaled/nasal delivery provide alternative routes of administration for macromolecules.

A new study from the United States National Institutes of Health (NIH) found that pairing the antidepressant amitriptyline with drugs designed to treat central nervous system diseases, enhances drug delivery to the brain by inhibiting the blood-brain barrier in rats. The blood-brain barrier serves as a natural, protective boundary, preventing most drugs from entering the brain. The research, performed in rats, appeared online April 27, 2017 in the Journal of Cerebral Blood Flow and Metabolism.

Catalent combines its formulation expertise, manufacturing excellence and particle size reduction technologies to provide a broad range of solutions in the development of inhalation and nasal drug products.

Companies believe that biologics and biosimilars would experience the fastest growth over the next year; there is also interest on increasing market penetration of generic drugs.

Sandoz, a Novartis division, announced that the Committee for Medicinal Products for Human Use (CHMP) has adopted positive opinions recommending the approval of its biosimilars rituximab and etanercept in Europe, for the same indications as the respective reference medicines.

In implementing quality by design for drug formulation, it is crucial to identify the critical properties of excipients and understand how their variation affects the final drug product.

The funding allows the company to broaden its range of services and finance its move to new laboratory facilities in the Illkirch-Graffenstaden innovation park in northeast France.

Pharmaceutical Technology spoke with CPhI North America presenter Jonathan Helfgott to discuss navigating GDUFA and helpful tips for submitting successful ANDAs.

The project will foster the translation of nanomedicine applications for the treatment of cardiovascular diseases.

The agency issued its recommendation for the influenza virus strains European vaccine manufacturers should include for 2017.

In a new study, researchers from Boston Children’s Hospital study responses to pneumococcal vaccine in infant monkeys.

Researchers continue efforts to overcome challenges of effective oral delivery of biologic drugs.

Carglumic acid is used in the management of rare, life-threatening inborn metabolic disorders affecting the urea cycle.
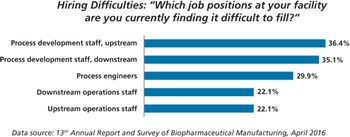
New study shows China biopharma companies face staffing shortages.

The transfer of fluids is governed by different equipment requirements across the medical, biopharma, and cell therapy manufacturing industries.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.
Design of experiment plays a crucial role in the optimization process of formulation development.

Interpretation of calorimetric data is tricky. The author suggests 10 questions that should be asked of any calorimetric method, along with the rationales behind them.

Solid dispersions based on organic polymers can have stability issues. Inorganic solids, especially those based on silica chemistry, may be suitable alternatives to organic polymers due to their pre-formed pore system, high absorptivity, and commercial availability in pharmaceutical quality. Mesoporous granulated colloidal silicon dioxide has been studied with class II and IV actives of the Biopharmaceutics Classification System for its ability to improve dissolution. Using suitable formulation strategies, the dissolution of these APIs could be significantly increased. The absorption of poorly soluble APIs onto silicon dioxide can, therefore, be considered a viable formulation path to overcome solubility challenges.

Process analytical technology, based on monitoring particle size distribution and tracking coating thickness measurements in real time, can be used to predict the dissolution of polymer-coated multiparticulates.

A case study reviews the reformulation and scale up of high drug load prototype using wet granulation process for a model formulation.

The newly installed Harro Hӧfliger encapsulation unit expands Capsugel’s clinical- and commercial-scale capacity for dry powder inhalation projects.