
Manufacturing will be carried out at the Pfizer Newbridge, Ireland, facility, which is now part of Pfizer CentreOne’s contract manufacturing network.

Manufacturing will be carried out at the Pfizer Newbridge, Ireland, facility, which is now part of Pfizer CentreOne’s contract manufacturing network.
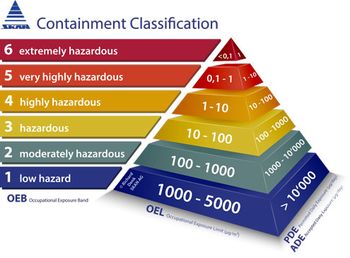
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Effective solutions for overcoming the high molecular weight, hydrophilicity, and instability of large biomolecules have yet to be identified.

Pharmaceutical Technology spoke with Frank Generotzky, plant manager for Baxter BioPharma Solutions’ Halle, Germany facility, about operational excellence at the site.

This study investigated the stability of solid lactose stored under high temperature and humidity conditions.

The authors discuss the challenges of capsule filling in preclinical and clinical studies.

Capsule filling is a complex process, and the product to be encapsulated must be well developed to ensure mass uniformity.

The UK Stem Cell Bank released validated stem-cell lines for researchers developing novel cell-based therapies for clinical trials.

Sanofi and Lonza formed a joint venture to build and operate a large-scale mammalian cell culture facility for monoclonal antibody production in Visp, Switzerland.

In 2016, FDA approved 630 ANDAs and tentatively approved 183 ANDAs, the highest number to date, according to the report.

The industry is becoming more consolidated, but there needs to be some strategy behind the mergers and acquisitions.

The authors discuss regulatory and patent issues with combination products.

Under the agreement, Abzena will manufacture magacizumab, an antibody created using the ‘Abzena inside’ Composite Human Antibody technology.

The trends in the year 2017 will present the industry with new challenges, and companies will be able to meet them successfully if they focus on the issues at hand.

The new commercial site, set to be located in Cambridge, Boston, MA, will serve clients on both the East and West Coast. It will also be the base for reaching new customers in the area.

The directorate says monographs are flexible and changeable and their compliance does not on its own determine biosimilarity in biosimilars.

Aurobindo has added four cell-culture derived biosimilars to its product line.

The Generic Pharmaceutical Association announces a rebranding campaign to expand access to medicines.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

Dr. Reddy’s has expanded its commercial operations in Europe with the introduction of its range of generic drugs in France.

Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.

This three-year partnership will explore and identify new tools and methods to modify and optimize the Chinese hamster ovary (CHO) cell line performance.

The investment will be used to advance Nemaura’s R&D programs, which include liquid vaccines that have been reformulated for administration through the skin using its micropatch drug delivery technology.

According to results from the FOURIER trial, Repatha significantly reduced the risk of cardiovascular events and death in patients with atherosclerotic cardiovascular disease.

Pump systems must be designed to meet the needs of specific processes, including preventing cross-contamination and damage due to shear forces.