
Average deal size and amounts invested increased in the second quarter, but totals trail funding of last year.

Average deal size and amounts invested increased in the second quarter, but totals trail funding of last year.

AAPS Global Health Focus Group's Kishor M. Wasan discusses new initiatives.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.

Could oral absorption-enhancing technologies change the shape of protein delivery?

The authors describe the concept of the limiting discriminatory the limiting discriminatory threshold (LDT) as an objective means of evaluating the inherent quality requirement of a large-sample content-uniformity test.

A Q&A with Rao Tatapudy, vice-president of scientific affairs at Catalent, on recent industry trends.

Manufacturers fund research and reduce prices to tackle diseases.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

In a 2004 guidance, FDA says that the "use of redundant sterilizing filters should be considered in many cases." But not all manufacturers agree on what redundant filtration is.

Representatives from FDA, the Generic Pharmaceutical Association, the European Fine Chemicals Group, and a task force from the Society of Chemical Manufacturers and Affiliates are nearing completion of draft legislation for the Generic Drug User Fee Act.

Powder Systems Ltd's (PSL's) new GFD™ isolator combines the latest innovation to provide a high containment lab scale filtration and drying solution with the same reliability and benefits as PSL high containment production size filter dryers.
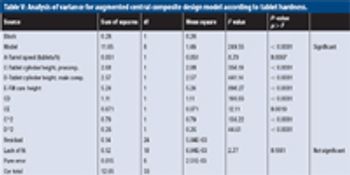
The author prepared and analyzed a detailed design of experiments for the manufacture of a simple tablet formulation. The aim was to test whether tablet hardness and weight could be controlled during the compression process by adjusting certain machine parameters.

A Q&A with John Plachetka, chair, president, and CEO of POZEN, on recent industry trends.

The EU debt crisis portends of possible negative repercussions for the dose CMO industry.

Might European officials reverse their position on acceptable production methods?

Reauthorization of pediatric exclusivity provisions looms in 2012 and debates begin anew.

International Federation of Pharmaceutical Manufacturers and Associations takes global action to improve public health.

Researchers at MIT and Harvard University report on new methods for producing microscale hydrogels.

Elham Blouet from Roquette explains the importance of carbohydrates for injection and the challenges in this niche market.
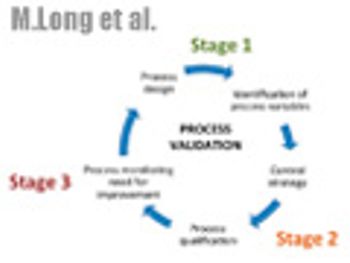
The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.

A Q&A with Joe Cascone, director of potent pharmaceutical development at Metrics, moderated by Patricia Van Arnum. Discussion of the key considerations made in facility design, equipment selection, and operations to achieve desired levels of containment.

Highly potent APIs (HPAPIs) represent a growing area of interest for the pharma industry. Mark Griffiths, CEO of Carbogen Amcis AG, explains why.

We're all familiar with traditional pills and medicines, but how about medicated chewing gum? Marc Ribe of Cafosa Gum explains how APIs can be incorporated into a novel dosage form that can aid patient compliance.

Clarifying GMPs for excipients used as actives.

Recently, there have been many innovations in the latest techniques and technologies in API purification. In particular, the trend to single-use systems has had a significant impact on processes.