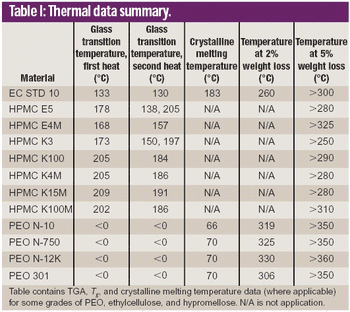
Hot melt extrusion (HME) formulation development depends heavily on choosing the appropriate polymers. This article reviews HME process parameters and highlights three polymers in HME: polyethylene oxide, ethylcellulose, and hypromellose.


Hot melt extrusion (HME) formulation development depends heavily on choosing the appropriate polymers. This article reviews HME process parameters and highlights three polymers in HME: polyethylene oxide, ethylcellulose, and hypromellose.
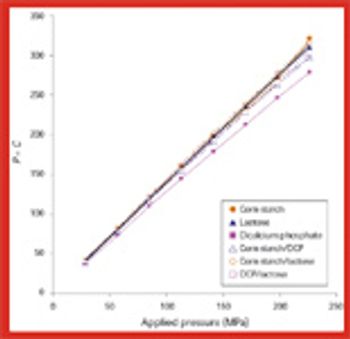
The survival of Bacillus subtilis spores in dicalcium phosphate, lactose, and corn starch and in their binary mixtures depends on the compressional properties of these materials and on parameters involved during the tableting process, including compression speed.
The previous studies on the incorporation of glyceryl monostearate into pellets by extrusion/spheronization has been extended to include a range of grades of this material plus a mixed medium chain partial glyceride and two glycerol esters of hydrogenated natural glycerides as described in this article.

Palatinit's "galenIQ" is line of multifunctional excipients that offer the combined advantages of other bulk excipients.
Making active pharmaceutical ingredients (APIs) requires long chains of chemical reactions and large quantities of solvents. Ask API manufacturers how they'd like to improve this process, and the responses are likely to be "make the reactions faster," "make the reactions cheaper," or "make the reactions more efficient." Then after all these economically driven answers, you might here, "make the reactions more environmentally friendly."

Chemical purity is the most important quality characteristic of a pharmaceutical substance. This article describes the latest scientific and technological advances to meet recent pharmacopoeial and regulatory requirements regarding the control of organic impurities in synthetically produced active substances. Future developments and suggestions for those working in quality control and raw material selection are discussed.

A polymer excipient that reportedly reduces spray time has received its first registration in a finished drug product.

Poly(ethylene oxide) is gaining the attention of research and development organizations and its application is extending into a wide range of drug delivery systems.
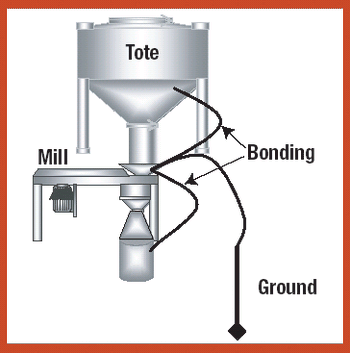
Various systems and measures can be used to safely handle and process explosive pharmaceutical compounds in a range of manufacturing procedures.
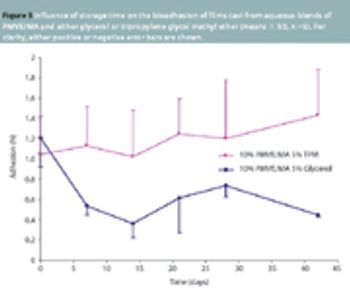
Bioadhesive films cast from aqueous blends of PMVE/MA have diverse uses, such as a means of establishing an electrically conducting interface for bioelectrodes and as an adhesive drug delivery matrix.

New Excipient Guidance Doesn?t Fill Regulatory Gap

The authors propose a strategy for classifying and validating inprocess testing methods.

The authors study the effects of sweet potato and cocoyam starches on the compressional and mechanical characteristics of a paracetamol tablet formulation using cornstarch BP.

Nanostructured lipid carriers are a new type of delivery system offering improved performance in terms of drug loading and long-term stability with the ability to form highly concentrated dispersions...

In this article, the author explains some of the technology behind using ion-exchange resins for drug delivery...

This article examines the importance of core design and formulation on the quality of a film coated tablet...

IPEC-Americas has just completed a major update to its significant change guideline to address current issues in the manufacture of excipient ingredients and to assist manufacturers in developing an impurity profile.

International Specialty Products (ISP) and Niro A/S have formed an alliance to develop and market spray drying and formulation technologies...

This article looks at how to adopt a systematic and prospective approach in the API development process to achieve documented, controlled synthetic processes...

European Directive 2004/27/EC will have massive repercussions on the manufacture and marketing of APIs and some excipients across Europe...

The authors examine the effects of three superdisintegrants on the dissolution and absorption of tenoxicam from solid-dispersion formulations.
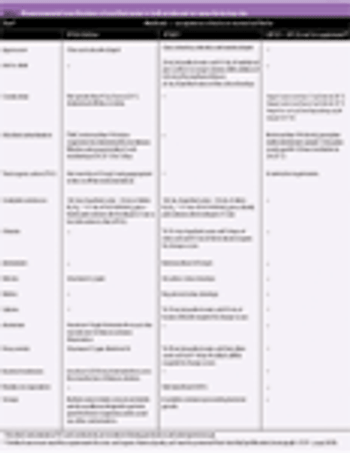
A harmonized global specification is possible providing that the procedures and acceptance criteria defined are acceptable to regulatory authorities in all regions.


With creative engineering and sophisticated chemistry, industry scientists offer big solutions for attaining small particle sizes and narrow distributions.

By enhancing stability and in vitro drug-release characteristics, limonene enantiomers are a natural-source alternative for developing and characterizing self-nanoemulsified drug delivery systems.