Experts describe best practices for sterility assurance in parenteral drug manufacturing. This article contains bonus online-exclusive material.

Experts describe best practices for sterility assurance in parenteral drug manufacturing. This article contains bonus online-exclusive material.

Russell Madsen, group leader of the Parenteral Drug Association (PDA) Filtration Interest Group, discusses technical and regulatory considerations in filtration for parenteral drug manufacturing

The growth in biologics drugs and vaccines is leading to greater demand for prefilled syringes, which provide greater safety and convenience for healthcare workers and patients.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

PharmTech speaks to Ray O'Connor from the National Institute for Bioprocessing Research and Training (NIBRT) for an overview of aseptic processing.

Even when all is well at the facility, one must expect the worst while braving the elements.

Experts share insights into analytical tools and techniques.

GSK Extends Deadline for Tender Offer to Acquire Human Genome Services; Sartorius Opens New Filter, Aseptic-Bag Production Facility in Puerto Rico; and More.

Experts share how to choose analytical tools and techniques when scaling up a lyophilization process.

How to avoid invisible and airborne contamination.

Closed-vial technology is an alternative to traditional glass vial filling that reduces the risk of contamination for the patient, simplifies the filling process, and provides easier handling for healthcare providers.

FDA has released a final guidance describing cGMP for preparing media fills for validation of aseptic preparations for positron emission tomography drugs.

Experts discuss the best practices for developing a QbD-based lyophilization process.

There are no two completely identical freeze dryer units in operation anywhere.

Lyophilisation is often necessary for pharmaceutical products to improve stability or shelf-life. However, the process can present difficulties, particularly when scaling up from the laboratory to commercial production. We bring experts together to discuss best practices for developing a lyophilisation process, including quality by design (QbD) and design space.
The author discusses the relative advantages and disadvantages of lyophilization in vials and dual-chamber systems.

Contamination is almost always related to human error and there is a clear drive to reduce human implications in aseptic operations. This can be achieved in multiple ways.

We have changed the brand of our stoppers for a product that we freeze-dry in vials. Since the change, we have observed a significant increase in rejects for collapsed cakes. Why are the cakes collapsing? What can we do to prevent this problem?

The future, and increasingly the present, for aseptic operations is isolated robotics. Companies that wish to gain competitive advantages in their operations are stepping up and taking notice.

In our manufacturing process, we are running into issues with our vial stoppers clumping in the feeder bowl. How can we ensure that the stoppers go through smoothly without clumping up?
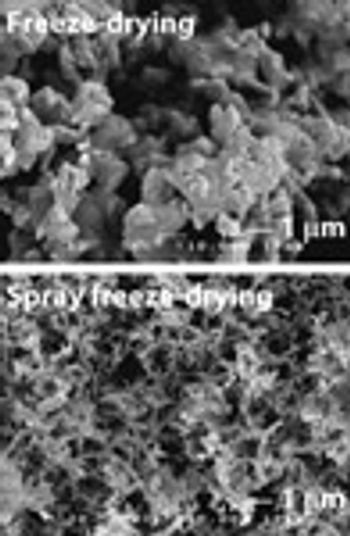
The authors discuss the preparation of lipophilic drug nanocrystals by controlled crystallization during freeze-drying.
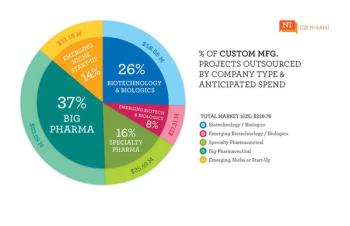
The article examines the drivers of customer perception of contract service providers of pharmaceutical development and manufacturing.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2011 edition from Meissner and Telstar.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

A technical forum moderated by Patricia Van Arnum