
Parallel trade has frustrated pharmaceutical manufacturers for years and now evidence has linked such trade to drug shortages in Europe. Parallel trade representatives have yet to respond, but will need to react quickly to salvage their reputation.

Parallel trade has frustrated pharmaceutical manufacturers for years and now evidence has linked such trade to drug shortages in Europe. Parallel trade representatives have yet to respond, but will need to react quickly to salvage their reputation.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2012 edition from EMD Millipore and Meissner Filtration Products.

Time- and cost-savings benefits help drive increased use of single-use, disposable systems in biopharmaceutical manufacturing.

An understanding of the pan-coating process based on first principles can support successful scale up.

Novartis, GlaxoSmithKline, and Lonza are among those participating in newly formed consortiums for developing and producing medical countermeasures.

European and US associations call for continued vigilance against the threat of counterfeit medicines.

In addition to the established coating systems (like the BFC and the BFC TriPan), Bohle developed a new coater to face the market needs, the Bohle-Tablet-Coater BTC.

Dr. Reddy's Laboratories and Merck Serono, a division of Merck KGaA, have partnered to codevelop a portfolio of biosimilar compounds in oncology.
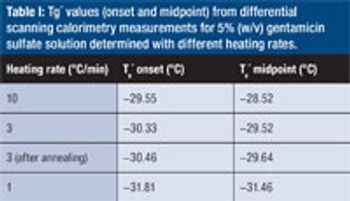
The authors evaluate the thermal properties of gentamicin sulfate as a small-molecule drug model in optimizing the freeze-drying cycle.

Peptides and related technologies to are starting to improve production.

New product reviews for June 2012.
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.
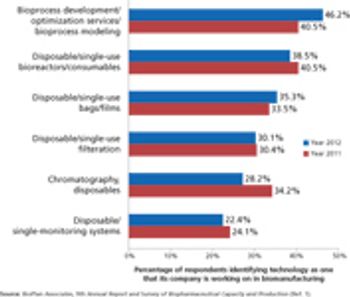
Industry wants more innovation, but can suppliers meet customer needs?

A Q&A with Larry Kadis, President and CEO of Federal Equipment, on recent industry trends.

Highlights included the latest in pharmaceutical packaging equipment, containers, and labels and new capabilities among contract service providers.

BIO is calling for a more patient-centric approach to user-fee reauthorization.

How to avoid invisible and airborne contamination.

US Pharmacopeia documents best supply-chain practices and seeks broad input on proposal.

New price-control policy has domestic and global firms waiting on the sidelines to launch products.

New coating technologies achieve high uniformity and reduce waste through mixing system advances and pan and airflow configuration.

UK chooses to use off-label drug indication to cut healthcare costs. Will others follow suit?

New legislation, government programs aim to bolster drug discovery and reduce regulatory hurdles.

Does global development have to entail multiple comparability studies?

Even the slightest of errors in exponential calculations can cause the biggest of headaches.

Nick Beckett from the UK law firm CMS Cameron McKenna explains how the UK government's proposed Patent Box legislation may impact the pharmaceutical sector.