
Properly identifying and classifying rouge, a form of surface corrosion that comes in many forms, is important in determining whether it may be affecting product quality and whether further actions are necessary.

Properly identifying and classifying rouge, a form of surface corrosion that comes in many forms, is important in determining whether it may be affecting product quality and whether further actions are necessary.

FDA issued a final rule on sterility testing on May 3, 2012, which amends the requirements for most licensed biological products and aims to provide manufacturers with the flexibility, as appropriate, to keep pace with technological and scientific advances. Many steps are changed or eliminated.

The UK's Medicines and Healthcare products Regulatory Agency has launched a new anticounterfeiting strategy with the aim of curbing the occurrence of falsified medicines in the county's supply chain.

A technical forum featuring Tim Freeman of Freeman Technology and Carl Levoguer of Malvern Instruments.

Manufacturers seek clear path to develop safe-use approaches for more risky OTC therapies.

It's better to catch costly mistakes in the laboratory before they reach the accounting department.
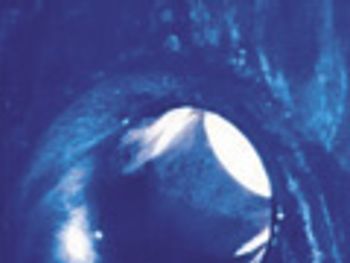
Equipment design and cleaning procedures both play a role in thorough sterilization and cleaning.

Industry experts working with extended-release injectables discuss challenges and solutions to formulating and manufacturing these complex products.

Apple's experience with manufacturing facilities in China present opportunity for future best practice.

New product reviews for May 2012.
The author discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.

Poland's government aims to make the Eastern European country a biotech powerhouse.

Advances in palladium-catalyzed hydrogenation, visible-light photocatalysis, and chemocatalyisis for heterocycles are some recent developments.

Industry, the public sector, and individuals can play an important role in creating solutions.
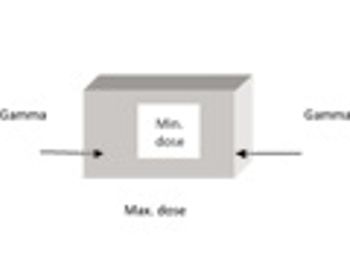
This paper examines the process of gamma irradiation of plastic materials used as part of single-use disposable systems in the pharmaceutical and biotechnology sectors, with a focus on validation requirements.
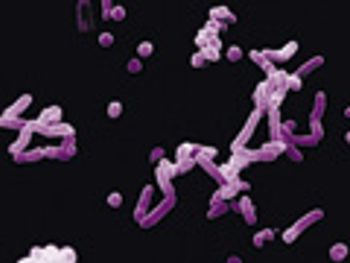
In this technical forum, experts describe different methods of rapid microbial testing and their applications.

As the French Presidential campaign is underway, and politicians collaborate with businesses to revive a flagging economy, the pharmaceutical industry seizes the chance to lobby for equitable taxation to give the sector a much-needed boost.

An analytical technique that is receiving increased attention in the pharmaceutical industry is atomic force microscopy. We interview Mark Leaper from the UK's De Montfort University to find out more about this technology.
The authors assert that the current gulf between aseptic processing and terminal sterilization can be bridged by re-examining fundamental regulatory philosophies for sterile-product manufacturing.

Closed-vial technology is an alternative to traditional glass vial filling that reduces the risk of contamination for the patient, simplifies the filling process, and provides easier handling for healthcare providers.

The authors provide a review of test methodology and standards, including current industry and regulatory proposals, for biological indicator growout times.

If a product does not have its own antimicrobial properties, then a preservative must be used to ensure microbiological safety.

There are various theories about how to scale up a solid dosage coating operation in a pan coater. This article provides a basic process understanding and scale-up theory based on first principles.
Readers share their views on bioprocessing challenges, equipment use, and outsourcing trends in our annual bioprocessing equipment and processing survey.

My network failed and I had to scrap my batch because my historian did not collect the required data. How do I upgrade the reliability of my network to maintain data continuity if my network fails again?