
Germany has shifted from a market where the pharmaceutical industry could enjoy considerable pricing freedom to a sensitive market influenced by cost-containment policies.

Germany has shifted from a market where the pharmaceutical industry could enjoy considerable pricing freedom to a sensitive market influenced by cost-containment policies.
An examination of the current and projected market for biosimilars, development costs for biosimilars compared with small-molecule generic drug, and partnerships in biosimilars.

The author discusses how a CDMO helps in gaining process understanding and in developing robust, high-quality products and processes.

The authors highlight costs, benefits, and implementation success factors across first, second, and third generations of facility management outsourcing contracts.

Annual survey shows strong growth for service providers and promises to continue into 2013.

Dedong Wu, senior scientist at AstraZeneca, explains how nanoparticle engineering and crystal engineering can aid solubility.

A report from the European Commission shows that fake pharmaceuticals were the top articles detained by European Union customs in 2011.

MedImmune, the global biologics arm of AstraZeneca, announced that it is restructuring its infectious disease and vaccines R&D and operations. This will result in the closure of MedImmune's sites in Mountain View and Santa Clara, California.

Experts share insights into analytical tools and techniques.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the July 2012 edition from Sartorius Stedim Biotech and Waters.

Continuous manufacturing of amorphous solid dispersions in a twin-screw extruder is well-suited to quality by design processes such as defining design space.

GSK Extends Deadline for Tender Offer to Acquire Human Genome Services; Sartorius Opens New Filter, Aseptic-Bag Production Facility in Puerto Rico; and More.

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.

Experts share how to choose analytical tools and techniques when scaling up a lyophilization process.

Industry experts share their insight on solid-dosage and sterile manufacturing.

Tracking changes from spinoffs of chemical companies to life-sciences powerhouses.

EMA and MHRA provide insight into increased GMP deficiencies.

Careful attention to detail will help to prevent valuable assets from "melting" away.

A Q&A with Thomas E. D'Ambra, Chairman, CEO, & President of AMRI, on recent industry trends.

Taking time to appreciate the industry's greatest achievements will inspire growth ahead.
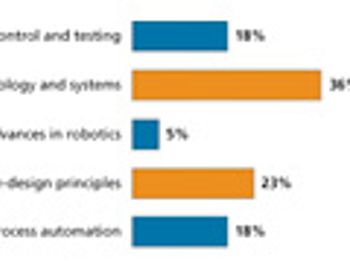
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Developments in hot-melt extrusion using twin-screw extruders to make solid-dosage drug forms.

An industry roundtable on how users and makers can best assess and manage black specks.