
Foreign firms struggle against stricter patent laws, but all is not lost.

Foreign firms struggle against stricter patent laws, but all is not lost.

Real-time experimentation may offer continuous process improvement.
Today's pharmaceutical companies are striving to reduce costs and maximize efficiencies, and must make decisions on the best way to deploy their limited resources.

Only the strong survive when it comes to pharmaceutical packaging and shipping.

The authors examine the use of various grades of direct-compression mannitol in direct-compression tableting process to evaluate the content uniformity of micronized APIs and excipients in a solid-dosage formulation.

A Q&A with James Ingebrand, Vice President and General Manager of 3M Drug Delivery Systems Division, on recent industry trends.

Particle-engineering technologies, such as crystal design for crystallization and producting cocrystals, particle-size reduction, and amorphous solid dispersions, help to optimize delivery of a drug.

Fluid-Bag Ltd provides comprehensive 900 and 1000 litre flexible IBC systems for liquid and semi-solid products, including filling and discharging equipment. The GMP compliant container system is designed to guarantee uniform liquid, maximise payload and minimise discharge residue (0.5% residue).

Working together affords many unseen opportunities for pharmaceutical innovation.
Fixed-dose combination drug therapies give rise to innovation in solid-dosage formulations and manufacturing.

Measuring the size of the market for contract manufacturing services requires a careful hand.

Q&A with David Elder and Richard Wright of Strategic Compliance Consulting, PAREXEL International. Both Elder and Wright formerly served with FDA.

The physical form of an API is an important consideration in formulation development. Particle-engineering technologies, such as crystal design for controlling crystallisation and producing cocrystals, particle-size reduction and amorphous solid dispersions, help to optimise delivery of a drug.

Watson Laboratories has recalled two lots of hydrocodone bitartrate and acetaminophen tablets.

Foaming can be used to improve processing in the extruder without the use of a plasticizer, facilitate milling, increase dissolution rates, and produce novel dosage forms.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the September 2012 edition from Uhlmann Packaging Systems and GE Healthcare.

Critical process parameters must be considered to optimize manufacturing of topical dosage forms.

Bristol-Myers Squibb has initiated a voluntary recall of 10 lots of BiCNU (carmustine for injection) previously manufactured by Ben Venue Laboratories, a former, third-party contract manufacturer for the company, to the user level.

Generic-drug manufacturers are preparing to pay fees to FDA for the first time in the agency’s history.
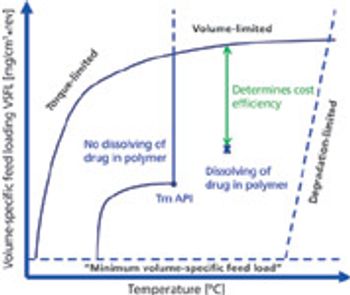
The advantages and disadvantages of hot-melt extrusion in solid dispersion formulations.

Q&A on GDUFA implementation with Aloka Srinivasan, PhD, a principal consultant with Parexel and former team leader in FDA's Office of Generic Drugs.

A review of taints and odors in the pharmaceutical and consumer healthcare industries.
IQ Consortium representatives explore and define common industry approaches and practices for applying GMPs in early development, with a focus on stability.

Packaging and monitoring tools protect temperature-sensitive pharmaceuticals.

Ties between the biotechnology industry and university research are crucial.