
Brazil takes first steps towards gaining quality requirements for pharmaceutical excipients.

Brazil takes first steps towards gaining quality requirements for pharmaceutical excipients.

MIT survey results address product and site characteristics that statistically correlate with quality performance.
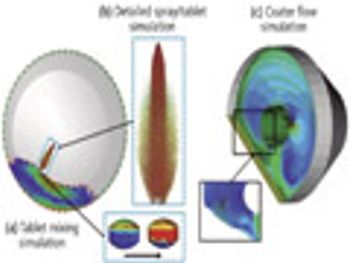
The authors investigate the tablet-coating process using a combination of different simulation techniques.

The authors describe a solid form technology platform used to optimize salt selection, cocrystallization identification and modification, or the development of a free form.

Pfizer has two manufacturing facilities in Germany for high-potency manufacturing, respectively in Freiburg and Illertissen. Pharmaceutical Technology's Executive Editor Patricia Van Arnum visited the facilities and spoke to the company about the design and operation of these facilities.
There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

A Q&A with BASF moderated by Patricia Van Arnum.

Rob Blanchard and Clive Roberts discuss the issues surrounding tablet sticking.
The authors explain chemical transformations that are achievable through certain biocatalytic routes.

An industry roundtable representing Metrics, Cambrex, Carbogen Amcis, Euticals, Ferro Pfanstiehl, and SAFC.

This article presents an overview of ISPE's guide on project management.

The author discusses the key provisions of GDUFA as they relate to the pharmaceutical supply chain, including parity of inspections between domestic and foreign sites for both finished dosage forms and APIs of generic drugs.

The International Society of Automation’s (ISA’s) 7th Marketing and Sales Summit, held Aug. 15–17, 2012 in Austin, Texas, was themed “New Rules of Customer Engagement: Riding the Winds of Change”, and emphasized the need to adapt to the changing needs, expectations, and behaviors of marketplace decision makers, according to an Aug. 27, 2012 press release.

The European Medicines Agency has recommended that the anticancer medicine DepoCyte be recalled from EU countries following the discovery of manufacturing deficiencies at Pacira Pharmaceuticals' San Diego site.

Pfizer and Mylan have agreed to establish an exclusive long-term collaboration to develop, manufacture, distribute, and market generic drugs in Japan. The products included in the collaboration are expected to be sold under the Pfizer brand with joint labeling.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2012 edition from Getinge and MG America.

Service agreements, expert liaisons, and scorecards are important to consider.

Recycling is becoming a viable option for disposal of single-use systems.

A Q&A with Babu Padmanabhan, Managing Director and Chief Knowledge Officer of STEER Engineering, on recent industry trends.

Manufacturers willing to report bad news about the supply can help reverse the shortage trend.

After a series of government reforms that are appealing to both domestic and foreign players, the Japanese pharmaceutical market is making a comeback.

A look at elastomer changeout times shows how industry knowledge improves operations and cost.

Pharmaceutical Technology's annual manufacturing investment update shows slight gains in biopharmaceutical manufacturing and emerging markets and continued restructuring of supply networks.
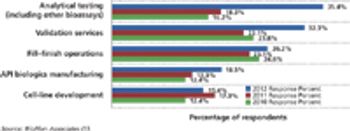
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

The authors compare direct combustion with rinse and swab sampling methods.