
Oostdyk discusses the latest industry developments and trends.

Oostdyk discusses the latest industry developments and trends.

Thanks to their keen observations, these auditors reveal the true culprits of deviations.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

Robotic systems provide flexibility and efficiency (and they're not as difficult to use as you think). This article contains bonus online-exclusive material.

An outstanding new book reviews alternative solvents with an eye to sustainable pharmaceutical processes.

A second-generation and green manufacturing process for testosterone provided economic and ecological benefits.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Authenticating tools help identify counterfeit drug products. This article contains bonus online-exclusive material.
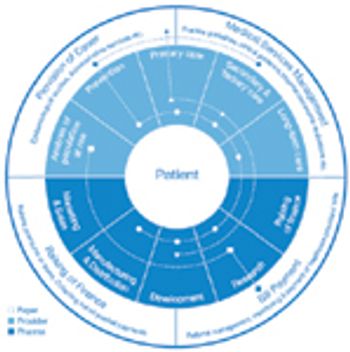
Personalized medicine and integrated healthcare delivery require new business and pricing models. This article contains bonus online-exclusive material.

Select contract manufacturing organizations roll out expansions for production of active pharmaceutical ingredients and intermediates.

Health crises generate support for new vaccines and treatments for diseases found in developing nations.

Pfizer uses green-chemistry in a second-generation manufacturing route for gabapentin.
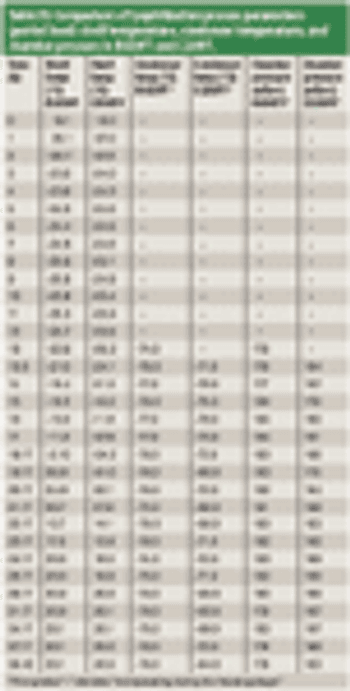
The authors describe a comprehensive methodology for establishing functional equivalence among various lyophilizers.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

Designated as a "pharmerging market," Brazil is revamping its pricing models.

The author describes the approach taken to develop a facility dedicated to handling potent and cytotoxic drug substances.
A Project Manager's Perspective.
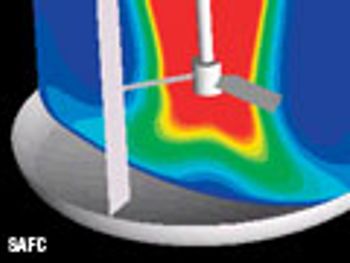
The authors discuss the advantages of microreactors and flow chemistry for various reaction types in achieving improved process economics and reaction efficiency.
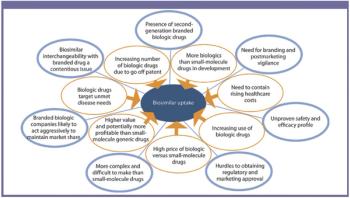
The author reviews the major biopharmaceutical markets' activity and predicts how the markets may evolve.

Thomas LaVake, manager, worldwide environment, health &safety at Johnson & Johnson, provides a perspective of sustainability practices for the pharmaceutical industry.
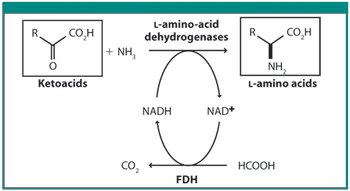
Scientists from DSM and Kaneka discuss various techniques in this roundtable moderated by Patricia Van Arnum.

Smoller talks about the benefits of targeted knockout rats.

Combination products have been researched and administered for many years, some successfully and others not.

Combining drugs with synergistic mechanisms of action yields some indisputable benefits; improved efficacy, reduced dosing, enhanced patient compliance, to name just a few.

Increase your chances of success in the biggest market in the world.