
Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

Roche has officially withdrawn from the Pharmaceutical Research and Manufacturers Association (PhRMA) after being a member for 36 years.

Johnson & Johnson has agreed to pay $1.0 billion to acquire the assets and rights of the Alzheimer's immunotherapy program of the biopharmaceutical company Elan, form a new company with Elan based on the AIP program, and gain an 18.4% stake in Elan.

The Federal Coordinating Council for Comparative Effectiveness Research recommended that data infrastructure be the primary investment for the US Department of Health and Human Services's (HHS) comparative-effectiveness research (CER) funds.

Advances in micellar catalysis, solid-state chemistry, catalytic asymmetric synthesis, and function-oriented synthesis for natural products represent noteworthy developments in green chemistry that can be applied to the synthesis of active pharmaceutical ingredients.
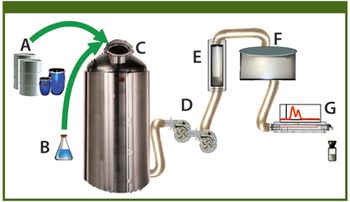
The author provides a review of PAT and tools such as near infrared analysis that may facilitate the use of PAT in the biopharmaceutical sector.

A joint biopharmaceutical manufacturing facility in India by Kenwell and Boehringer Ingelheim ushers in new era.

Formulators and manufacturers have many options for modifying release profiles in multiple-API products.

On June 25, US Marshalls seized all drug products and ingredients at three facilities of Caraco Pharmaceutical Laboratories.

The UK's Medicines and Healthcare Regulatory Agency (MHRA) has published the outcome of a consultation on measures to strengthen the country's drug supply chain.

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...
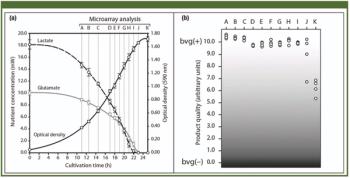
This case study describes the implementation of process analytical technology on the cultivation process step of a whole-cell vaccine against whooping cough disease.

Pan coating has been the preferred method of coating tablets for more than 20 years, but core coating is becoming more popular.

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

REMS to improve the safe use of opioids may lead to controls on other high-risk medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.

Dramatic increases in yields from cell lines and the increasing popularity of single-use technologies will change the way we look at future bioprocessing facilities and process designs.
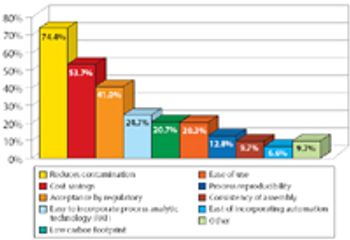
A Pharmaceutical Technology report looks at trends in biopharmaceutical manufacturing. This article contains bonus online-exclusive material.
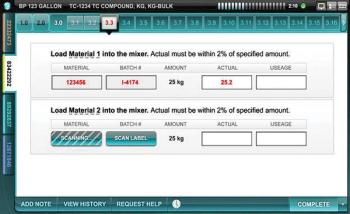
Editors' Picks of Pharmaceutical Science & Technology Innovations

While regulators begin to address nano-based drugs, industry should get its risk data ready.

Some GMP agents seem to find a way to squander time, money, and common sense.

Is it good policy to pay for bad behavior?
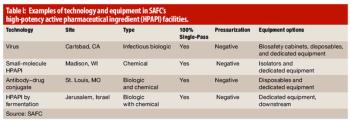
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

Brief pharmaceutical news items for July 2009.

A look at the true cost-drivers of cell-culture production.