
Lonza (Basel) has submitted a non-binding proposal that would see it acquire all of the Restricted Voting Shares of US-based Patheon.

Lonza (Basel) has submitted a non-binding proposal that would see it acquire all of the Restricted Voting Shares of US-based Patheon.

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes called the "cruise ship virus."

Researchers at the J. Craig Venter Institute (JCVI), a genomic research organization, reported that they successfully transformed one bacterium into a different strain by transferring the entire bacterial genome of the first strain into a second, related strain of bacteria.

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Researchers from a university in the US are urging the FDA to demand that drug manufacturers state how new medications compare with similar treatments on product labels.

Amcor (Australia) has made an offer of $2025 million for parts of Alcan Packaging (France), including the company's global pharmaceuticals division.

NIR Prediction of Solid Dosage Form Dissolution Profiles - Foss Whitepaper

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

Researchers from the Stanford University School of Medicine in California are urging the US Food and Drug Administration to demand that drug manufacturers state how new medications compare with similar treatments on product labels.

India accounts for approximately 3% of the global outsourcing market, which indicates a significant opportunity for growth, according to a report published by Ernst and Young and the Organization of Pharmaceutical Producers of India.

An initiative to develop consensus-based industry standards to identify and define green chemicals and process technologies is underway.

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

Manufacturing methods for new drugs could be made greener and more efficient with the help of marine microbes.

The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.
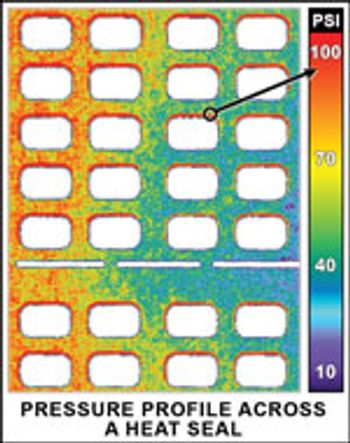
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the August 2009 edition from AEC and Sensor Products.

Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.

On August 6, the Biotechnology Industry Organization (BIO) filed an amicus brief asking the US Supreme Court to overturn Bilski v. Doll, a decision of the US Court of Appeals for the Federal Circuit.

The US Food and Drug Administration released its Guidance for Industry titled Pharmaceutical Components at Risk for Melamine Contamination.

Soelkner discusses the latest industry developments and trends.

Collaborative planning and execution among clinical research, clinical operations, and supply-chain managers are key elements in effectively managing an increasingly complex and global supply chain for clinical trial materials.

The US Food and Drug Administration announced its prescription drug user fee rates for fiscal year 2010 in the August 3 Federal Register.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...
Editors' Picks of Pharmaceutical Science & Technology Innovations