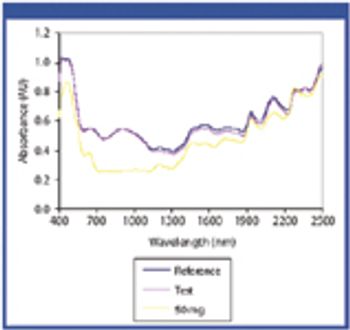
The authors applied near-infrared (NIR) spectrophotometry to assess whether eight drug products were authentic or counterfeit.

The authors applied near-infrared (NIR) spectrophotometry to assess whether eight drug products were authentic or counterfeit.

The author of a book about biopharmaceutical production includes irrelevant information.

Individuals and companies at the top seem to have no problem short-circuiting their success.

Determined to prevent further supply-chain breaches, industry takes charge, offers proposals.

Pressure to reduce healthcare spending has put drug rebates, price cuts, and tax hikes on the table.

An ounce of contamination usually leads to a mountain of investigation.

In the aftermath of recent restructuring, Big Pharma is sporting a reduced global manufacturing footprint while intensifying its focus to biologics and emerging markets. What will be the look of tomorrow's manufacturing networks?
View the corporate lineages of pharma's top companies.

Industry is taking a step forward by addressing supply-chain integrity through the formation of Rx-360. PhamTech's podcast explores the subject.
The contract services industry may not be as robust in 2009 as it has been in previous years, but it's not as bad as many people think.
The author provides advice about evaluating contract analytical laboratories and establishing an effective procedure for working with them to perform reliable stability studies.

This week, Sanofi Pasteur, the vaccines division of sanofi aventis (Paris), acquired the ShanH subsidiary of Mérieux Alliance, a holding company.

The number of alliances between diagnostics companies and pharmaceutical companies is set to rise because of the growth of personalized medicine, according to a report from PricewaterhouseCoopers.

In a move to strengthen development of its biologics portfolio, Bristol-Myers Squibb (BMS, New York) has agreed to acquire the biopharmaceutical company Medarex (Princeton, NJ) for $16 per share or approximately $2.4 billion.

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

The United Kingdom's pharmaceutical sector sector will soon have its own organizational body to help employers drive the key skills agenda of the industry.

China's State Food and Drug Administration has completed a new draft of GMP guidelines, but after a series of quality-control events, it will take time for the country to regain the global pharmaceutical industry's trust.

Last week, Rep. George Miller (D-CA), Rep. Charles Rangel (D-NY), and Rep. Henry Waxman (D-CA), introduced the America's Affordable Health Choices Act (H.R. 3200) to reform the nation's healthcare system.

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Devices that measure relative humidity (e.g., sensors and transmitters) play a relatively small role in cleanroom management, but their failure can cause significant problems. Operators should bear several factors in mind to ensure that sensors function properly and maintain the appropriate humidity.

Drugmakers have the common goal of manufacturing safe and sterile pharmaceutical products and understand that the filtration process is a critical means of achieving this goal. Preuse filter-integrity testing provides evidence that a filter will perform correctly, has the right pore size, and has been installed correctly.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the July 2009 edition from Mettler Toledo Safeline and Munters.

An interview with Trevor Perrior, Research Director at Domainex. Domainex is currently collaborating with St George's University of London and the University of Manchester in the hope of developing a better treatment for asthma.

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

In the wake of Dow Chemical?s announcement that it is to close facilities in the UK, the country?s biggest union, Unite, has called for urgent intervention from the Government to avert, what it believes, could be a crisis in the UK chemical sector.