
A review of advancements in areas such as active ingredients, formulation, technology, regulation, and analytical testing.

A review of advancements in areas such as active ingredients, formulation, technology, regulation, and analytical testing.

Fundamental validation skills related to heating, ventilation, and air conditioning must be thoroughly understood. Knowing the early history and development of validation is essential to understand the current set of regulations and standards governing the industry. This article reviews the history and outcome of contamination in large-volume parenteral drug products and discusses the qualification requirements of modern HVAC systems.

While recapping the past 30 years' innovations in pharmaceutical technology, it's also important to start preparing for the next 30 years. But staying ahead of the curve will be challenging.

Automation took hold gradually in the life-science industry. Its adoption brought the industry innovations and improved efficiency. Recent years witnessed the emergence of batch-automation systems and the development of standards for automation. The authors discuss the major changes automation brought to the industry and examine the rapid pace of technological development.

Bioanalytical Systems (West Lafayette, IN) extended the employment contract of its president and CEO, Richard M. Shepperd, through December 31, 2009. Shepperd has served as interim president and CEO since October 2006.

Thirty years ago, the world was a very different place; so was the pharma industry.

Sterile product manufacturing and related testing have evolved significantly during the last 30 years. From requirements for acceptance criteria for media-fill tests, to developing validated approaches for moist-heat sterilization, to the introduction of formalized sterility-testing practices, the pharmaceutical industry has made significant advances in testing and in key technology such as isolators, prefilled syringes, automation, and robotics. The author outlines the key regulatory and technical changes to sterile product manufacturing and takes a visionary look for the next era of sterile manufacturing marked by a greater emphasis on risk analysis.
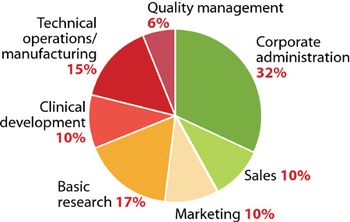
Trenton, NJ (May 18)-Pharmaceutical and medical technology companies in New Jersey have found a striking disparity between six high-demand occupations and the number of qualified workers to fill those positions, according to a report issued by the HealthCare Institute of New Jersey (HINJ). Modest job growth in this field is expected for the next four years, states the report.

Traditionally, in pharmaceutical water systems the main method for controlling bacterial levels was heat or chemical sanitants...

Drug companies have come to realize that spending heavily on creating new blockbuster drugs is risky and less cost-effective ...

Driven by a rapidly ageing population and a known high consumption of pharmaceuticals, the French pharmaceutical market has always appeared buoyant.
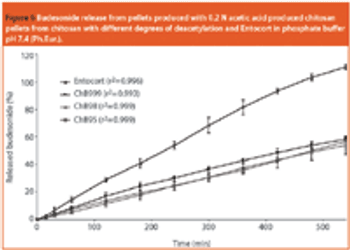
Chitosan is of pharmaceutical interest because of its positive attributes with respect to toxicity, biocompatibility and bioavailability.

Double-digit growth is projected for the US generic drug market, and the industry positions for opportunities in biosimilars.

Pfizer CentreSource is proceeding with a new plan for the supply of steroids and steroid intermediates through a recently signed pact with two Asian contract manufacturing organizations.

Washington, DC (June 14)-The US Department of Health and Human Services awarded two contracts totaling $132.5 million to Sanofi Pasteur and MedImmune to retrofit their influenza vaccine manufacturing facilities.

The largest study of the genetics behind the world?s most common diseases has been deemed as the start of a new era in medical research.

Brussels (May 30)-The European Federation of Pharmaceutical Industries and Associations (EFPIA) called for the pan-European and industry-wide adoption of 2-D barcode technology to combat the increase in counterfeit drugs in Europe.

WHO is working with vaccine manufacturers to move ahead on plans to create a global stockpile of vaccine for the H5N1 avian influenza virus.

Kvistgard, Denmark (June 4)-Bavarian Nordic received a $1.6-billion contract from the US Department of Health and Human Services to manufacture and deliver 20 million doses of its smallpox vaccine "Imvamune."

Rockville, MD (May 31)-The US Food and Drug Administration finalized guidances for seasonal and pandemic influenza vaccines, outlining the regulatory pathways for developing and approving these products.
Pharma companies and CMOs build positions in mAbs.

News and Views
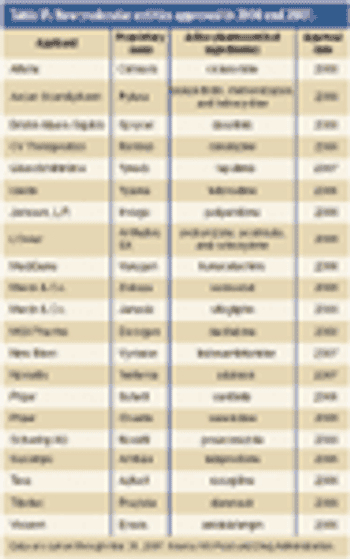
Pharmaceutical Technology's Annual Manufacturers' Rankings provides perspectives on revenues, product positioning, R&D spending, pharmaceutical manufacturing activity, and capital projects of the major drug companies.

While some companies float with the tide, others grab market share while business is good.

Exhibitors' products prevent counterfeiting, provide child resistance, protect product quality, and improve packaging-line efficiency.