
Able Interim Chief Resigns Amid Drug Recall

Able Interim Chief Resigns Amid Drug Recall

First Contraceptive Spray Offers Advantages over the Pill

Q Chip Develops Fully Functioning MicroPlant
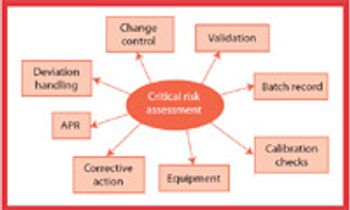
A stepwise, process risk-assessment approach can facilitate the identification and understanding of critical process parameters, quality attributes, and in-process controls. This approach can lead to more use of science- and risk-based regulatory practices to simplify the regulatory requirements for changes to synthetic processes and to support the underlying quality systems that ensure compliance.

Singapore is competing aggressively with India and China for a piece of the Asian sourcing business.

Can macromolecular processes learn from small-molecule experience? Burdened by exploding bioreactor productivity, architects of downstream bioseparation technology are looking into the drug industry's past for inspiration, while small-molecule companies adopt techniques pioneered by biotechnology. (The first of three articles on the current state of separations.)

Genentech Anticipates Full-Capacity Manufacturing

Schering AG To Cut Plants and Staff

Molding Technique Improves Nanoparticle Production

Warning Letter: Pragmatic Materials

FDA Posts Q5E Biotech and Biological Products Comparability Guideline

Blister Packaging Trumps Bottles For Patient Compliance, Study Shows

Wyeth and Purdue Announce Restructuring Plans

Development Collaboration for Ophthalmic Drugs Could Eliminate Injections

Warning letter: C&M Oxyfill

Self-Assembly Nanotechnology Improves Microencapsulation

Eli Lilly Scales Back Planned Virginia Insulin Plant

Officials at the US Food and Drug Administration are working with industry and academia to develop more efficient and reliable drug production processes that can ensure a consistent supply of high-quality therapies. A modern manufacturing system based on harmonized regulatory policies across global regions is critical for meeting public demand for safe and effective medicines, while also reducing production costs and eliminating waste.

Once considered mainly an afterthought in a company's lifecycle-management strategy, controlled-release dosage forms are now positioned at the forefront of many formulation strategies. In contrast to drug discovery, formulation work focuses not only on the intricacies of the active pharmaceutical ingredient (API), but also on fine-tuning the excipients, the release profile, and the delivery mechanism to provide optimal therapeutic benefit. Because of their wide range of applications and functionalities, especially in controlled-release therapies, polymers are among the most widely used excipients.
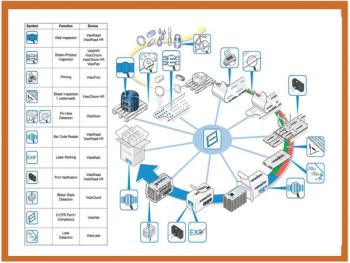
Most experts recommend layering protective technologies by selecting a combination of overt and covert techniques.

Purdue?s RFID Pedigree Program Enters Pilot Phase

Wet granulation is a size-enlargement process in which a liquid is used to achieve the agglomeration of solid particles. Agglomeration improves particles' tableting properties by rendering them free-flowing, nonsegregating, and suitable for compression (1).

Detection and identification of different polymorphic forms is, therefore, important throughout the drug development and manufacturing process.

Possible cross-contamination issues should be eliminated at the early stage of the project. The project sponsor should ensure that all relevant personnel from the production, quality control, logistics, and maintenance departments, as well as engineering, are involved in the conceptual stages of a design.

The use of quality management software (QMS) to automate manufacturing quality processes quickly is becoming an industry-wide initiative. Companies are turning to commercial off-the-shelf systems (COTS) to simplify implementation and validation efforts. Regardless of the system selected, the automation of these critical quality processes is subject to electronic records and electronic signatures (ER/ES) requirements, as set forth in the 21 CFR Part 11 regulation (1). Therefore, companies must adopt a comprehensive but manageable approach to Part 11 compliance as they begin to automate the processes that support product quality and efficacy to ensure they meet the requirements of good manufacturing practices and all predicate rules.