
American Pharmaceutical Partners to Merge with American BioScience

American Pharmaceutical Partners to Merge with American BioScience
Almost all pharmaceutical manufacturing processes require handling and processing cohesive powders. The application of sufficient shear (i.e., the total deformation that the bulk of granular material undergoes under applied shear stress) is an essential factor in such processes. Sufficient shear is required to mill and de-lump materials, achieve sufficient flow, and homogenize cohesive ingredients. Shear mixing plays a critical role in the blending of dry powders, particularly for those that contain a minor cohesive component such as a solid lubricant or a drug. This mechanism is necessary to achieve a satisfactory homogeneity and disintegrate possible agglomerates. Excessive shear can be disadvantageous, however, and can lead to electrostatic buildup, attrition, and overlubrication.
Solid oral drug products are one of the oldest of all manufactured dosage forms (1). Today, the development of an appropriate formulation of drug and excipients and of an effective manufacturing process to create a tablet or capsule is slowly transforming from a practice of applied art to one of applied science. The US Food and Drug Administration supports this change by expecting sponsors of new drug applications to understand, describe, and control materials and processes as well as the risks associated with drug product manufacturing (2). These steps will ensure the consistent production of products that meet their specifications and remain safe and effective during their shelf life.

The potentially huge demand for vaccines in light of the growing avian flu crisis...

Pfizer to Shut Parsippany Plant

Adjuvant for HPV Vaccine Enhances Immune Response Levels

Chiron Looks to Flu Vaccine Cell Culture; FDA Gears Up

Continuous manufacturing processes?little used in the pharmaceutical industry but the norm in oil, food, chemical, and polymer manufacturing?go hand-in-hand with the current emphasis on quality-by-design and automated process monitoring and control (aka, process analytical technology, PAT).

The process analytical technology (PAT) initiative has been percolating at the US Food and Drug Administration for a long time, explained FDA's John E. Simmons at the AAPS Annual Meeting and Exposition on Wednesday. "If you think of PAT as an isolated set of applications, I think you are missing the point," Simmons said. "The FDA would like PAT to become commonplace?not to be an initiative, but common practice."

This week, the US Food and Drug Administration posted an Oct. 20 Warning Letter (http://www.fda.gov/foi/warning_letters/g5567d.htm) sent by its Minneapolis, MN district office to Diversified Manufacturing Corporation (Newport, MN).

Control Development's (South Bend, IN, www.controldevelopment.com) blend uniformity and dryer monitor consists of a NIR spectrometer, a sampling head, computer, and software.

Generally, tablet and capsule film coatings are applied as aqueous or organic-based polymer solutions or dispersions, graduate student Sagarika Bose (University of Connecticut) explained during her Tuesday AAPS Graduate Student Symposium presentation, "Development and Evaluation of Solventless Photocurable Pharmaceutical Film Coating." However, organic film coatings can be flammable, toxic, and must comply with strict environmental regulations. Aqueous film coating can lead to the degradation of certain drugs by heat and water.
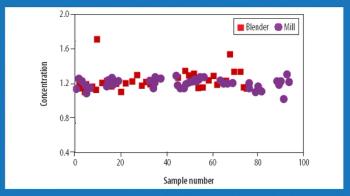
"The better we understand the relationship between process parameters and product attributes, the better control we'll have over product quality," said Beth Fowler, PhD, during Tuesday?s session on process monitoring at the AAPS Annual Meeting.

At the plenary session at the AAPS Annual Meeting, two researchers presented studies targeting completely different areas of stem cell research, but their work focused on the same ultimate goal: finding new therapies.

Pressures to save API are driving formulation developers toward smaller-scale laboratory processes, while pressures to save time put a premium on more- accurate laboratory scale tools.

"In my experience, you can generally tell where a person stands on the issue by the example he gives," said Art Mlodozeniec, PhD, a panelist at the Nov. 7 roundtable on follow-on biologics at the AAPS Annual Meeting in Nashville, Tennessee. "If he brings up human growth hormone and says the processes and impurities are easy to control, he's from the generic industry and supports approval for follow-on biologics. If he brings up the challenge of of erythropoeitin, he's from the innovator industry and opposes generics."

When it comes to developing a robust lyophilization process, formulators can "pay now or pay later," says Jeff Schwegman, PhD, founder and chief scientific officer for BioConvergence. Because 30% of new drugs in clinical trials are biotech-based therapeutics (compared with 7% 10 years ago), more than ever, the US Food and Drug Administration is paying close attention to lyophilization data and questioning pharmaceutical companies about their development cycles, especially cycle development transfer, shelf-temperature mapping, dryer-to-dryer comparison studies, formulation time, process validation, and cycle deviation. Consequently, this is pushing formulators to optimize formulation variables, conduct additional testing during early-stage development, and understanding critical process parameters, equipment qualifications, and manufacturing conditions that can influence formulation behavior at a large scale. Not taking the time or effort to achieve these goals during early development could lead to redundancies in formulation work - a reality observed too often in today's practices.

Thermo Electron (Waltham, MA, www.thermo.com) debuted the "LCQUAN 2.5" data-acquisition system at the AAPS Annual Meeting and Exposition on Monday. The new system expands the software offerings for the company's "Finnigan TSQ Quantum" series of triple quadrupole mass spectrometers.

"We must add our light to the sum of lights," declared Ron Reagan in his Nov. 6 keynote address to the 2005 Annual Meeting of the American Association of Pharmaceutical Scientists. He was quoting Billy Kwan, the half-Indonesian, half-Australian photojournalist of divided loyalties in the 1982 film, "The Year of Living Dangerously," a character who redeems himself by taking bold action in the face of moral crisis. Reagan encouraged the audience to take similar action to defend science, which he said is currently subordinated to political convenience.

Roche Puts Hold on Tamiflu in US

US District Court Rejects FDA Suit to Block Device Manufacturer

Novartis Purchases Chiron in a Total Buyout

The authors present a new approach to risk assessment for aseptic processing that emphasizes the contributions of personnel.
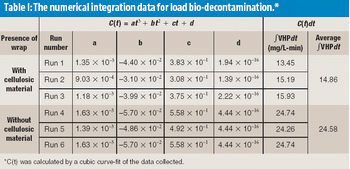
The authors focus on the effects of cellulosic materials during the performance qualification validation of a transfer barrier isolator used for the purpose of sterility testing.
Making active pharmaceutical ingredients (APIs) requires long chains of chemical reactions and large quantities of solvents. Ask API manufacturers how they'd like to improve this process, and the responses are likely to be "make the reactions faster," "make the reactions cheaper," or "make the reactions more efficient." Then after all these economically driven answers, you might here, "make the reactions more environmentally friendly."