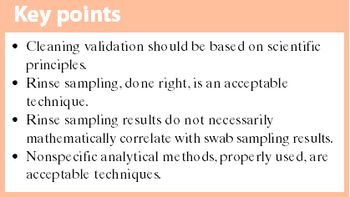
Performing studies to mathematically 'correlate' swab and rinse sampling values does not add any value. What's more, do not expect them to mathematically correlate.

Performing studies to mathematically 'correlate' swab and rinse sampling values does not add any value. What's more, do not expect them to mathematically correlate.

A range of insect exterminator equipment has been developed to improve the level of hygiene offered to the pharmaceutical and chemical manufacturing sectors. Berson's Insectron range uses ultraviolet (UV-A) and green light to attract flying insects, which are sensitive to these light sources.

Sigma-Aldrich Fine Chemicals (SAFC) has set up a new business division called SAFC Biosciences to encompass its biopharmaceutical development and cell culture-related manufacturing services.

Biomanufacturing managers believe the current lack of adequately trained personnel is one of the most serious problems facing the biomanufacturing industry.

Roche Plans New US Manufacturing Facility for Tamiflu while Cipla Prepares to Market Generic

Patheon, CEPH Begin Corrective Action Program for Omnicef OP

New 620 series pumps provide exceptional levels of performance, accuracy and output for biopharm manufacturers requiring low shear, hygienic fluid handling, with minimal downtime and long term cost savings. Flow rates are up to 18 litre/min and range features a choice of drives, two levels of PIN process protection and minimal downtime.

New Legislation To Ensure Stable Flu Supply

Design Exhibition, ?From Master?s Thesis to Medicine Cabinet?

Collaboration Targets Single-Shot Japanese Encephalitis Vaccine

Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
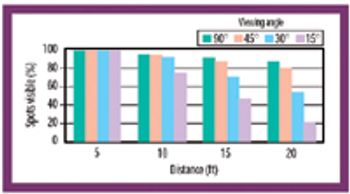
Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
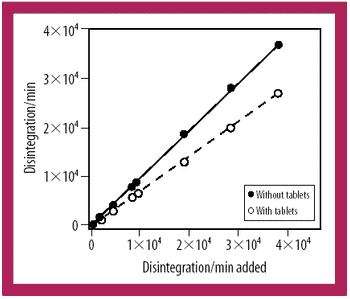
A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.

Although patient compliance problems have been receiving attention for at least a decade, many medications are still dispensed in bottles that contain a supply intended to last days or weeks and require considerable effort on the part of the patient or caregiver to keep track of the dosing schedule. As a result, when it comes to consistently taking the right dose at the right time for the duration of a prescription, many consumers don't do a very good job.

Two scenarios demonstrate the need to use the percent of parent drug loss rather than the percent of degradation products formed when reconciling mass balance calculations.

This review article discusses orally disintegrating tablets and their manufacturing technologies, development issues, and future trends.
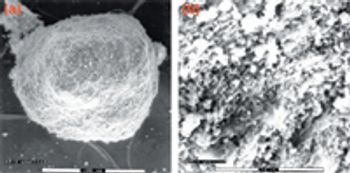
Although agitation improves drying efficiency and ensures uniformity of the final dry material, it can also affect the physical properties of the product as it dries. This study evaluates the effect of scale up and equipment selection on an active ingredient undergoing granulation during the drying process.

One of the world leaders in the manufacture of tablet compression tooling, I Holland, is offering a range of machines for the automatic polishing of tablet compression tooling.

In-process methods are key components of quality control in a chemical manufacturing plant. These methods ensure that a production reaction step conducted by trained operators within the entire validated process will produce a quality chemical entity in the expected yields. The presence of impurities and related compounds (derived from the reaction or secondary reactions) is a critical parameter that determines a synthetic material's quality.
Adding Braille to pharmaceutical packaging should be less of a challenge with the use of Esko-Graphics' Scope solution. EC Directive 2004/27/CE requires Braille labelling and information to be provided with pharmaceutical products for human use, and companies are scrambling to implement this by 31 October.

Picking may occur when granulation becomes imbedded on a punch surface and does not freely release when the compressing event has finished.

Dust extraction and centralized vacuum cleaning systems vary in their design, performance and costs. Different companies have different approaches to their design, however, there are some basic rules that must be followed if these systems are going to be immediately effective and avoid future problems.

The global pharmaceutical industry is currently in a state of flux according to Frost & Sullivan.

A CMO in Singapore manufacturing commercial biopharmaceuticals is the result of a partnership between two biopharmaceutical companies.

FDA Places All Andrx ANDAs on Hold