
Cephalon partners with hardware and software providers to test item-level tagging.

Cephalon partners with hardware and software providers to test item-level tagging.
Rapidly increasing cell-culture yields have thrown an increasing burden on downstream processes just as price pressures are pushing process developers to look for economies in every purification protocol. The time-honored, effective, and expensive war-horse, Protein A, is beginning to feel some competition from small-molecule mimetics.

Pharmaceutical science and technology innovation

Genecor Wins Vaccine-Development Contract

FDA Calls Chiron Facility Acceptable; GSK Gets Green Light For Flu Vaccine

Because disposable systems offer benefits that can impact the time for development as well as for the cost of production, the biotech sector is expected to continue to use these technologies ...

This article provides an overview of the important factors associated with air handling systems within pharmaceutical and biopharmaceutical facilities. It provides information on the need for these systems, design considerations and advice on the approach to commissioning and qualification.

Planning manufacturing capacity in the pharmaceutical industry is not for the faint-hearted. How can process designers help their clients to overcome some of the problems they face when planning to introduce new capacity? This article sets out to explain some of the techniques that are being employed in the early stages of project development.

Bavarian Nordic Sues Acambis for Patent Infringement

Acambis and Bavarian Nordic Vie for Smallpox Vaccine Contract

GSK Releases Flu Vaccine Production Strategies

US Government Orders 2M Doses of Avian Flu Vaccine
In 1987, when the US Food and Drug Administration issued its Guideline on General Principles of Process Validation, a young FDA reviewer asked her supervisors.

Solid dosage form manufacturers have long relied on shape and color as well as on-pill imprints of logos, product names, or numbers for product identification. But in these days of heightened counterfeiting concerns, the industry has a growing interest in adding more difficult-to-duplicate features to the pool of existing product identification techniques. Added security is particularly important on high-profile or high-cost drugs, as well as on pharmaceutical products supplied in bulk for repackaging.
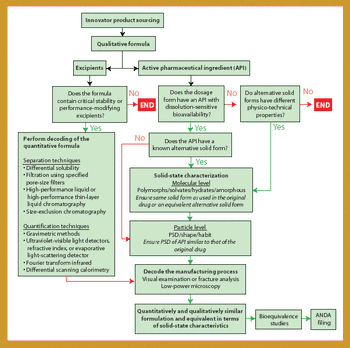
Being the first to gain the most is a fundamental principle in the generics business because several companies compete to create generics of successful products going off patent. For a generics company to maintain revenue growth in a market in which product prices continue to fall, it must secure a continuous flow of new products, with quality and speed to market being key drivers. Thus, generics companies must be highly skilled in product and process development (1), the generics business, and achieving bioequivalence-the most critical development area.

To help meet the needs of the fast-growing, global biopharmaceuticals industry, Pall Corporation has launched five innovative technologies aimed at increasing drug-manufacturing efficiency through customization and disposability. The Mustang XT5000 capsule is the company's novel chromatography technology for efficient capture of large molecules, especially used to develop DNA and virus-based drugs. Expanding its portfolio of technologies for disposable processing, Pall offers both the customizable Tangential Flow Filtration system, which incorporates single-use components for downstream processing applications, and the Kleenpak Connector — a new single-use device, which enables aseptic connections to be made instantly.
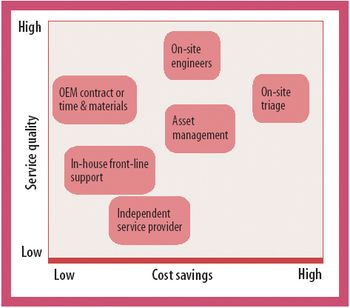
New maintenance models focused on efficiency improvements can deliver cost savings and enhanced service quality.

The first part of this article introduced the basic features of Raman spectroscopy and presented some examples of its application in the pharmaceutical industry. This second part focusses on the technique's application as a PAT tool within the pharmaceutical manufacturing environment. FDA's PAT initiative has provided motivation to explore the application of 'new' analytical technologies to the pharmaceutical manufacturing process and Raman spectroscopy shows great promise. The strengths and weaknesses of the technique as a potential PAT tool are discussed together with some examples of how this works in practice in a pharmaceutical manufacturing environment.

Measuring, understanding and ultimately controlling manufacturing processes offers pharmaceutical companies a route to greatly enhance the business effectiveness of both their product development process and facilities. PAT creates technological, business process and regulatory frameworks to enable this. This paper looks at a PAT overview within the context of developing and manufacturing a tablet product, highlighting the potential of FT-NIR spectroscopy.
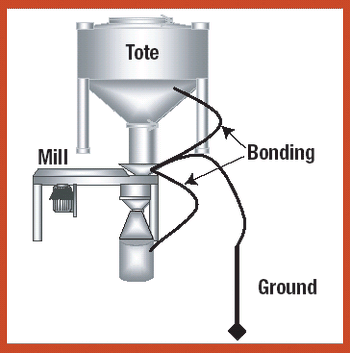
Various systems and measures can be used to safely handle and process explosive pharmaceutical compounds in a range of manufacturing procedures.

Process analysers have evolved from basic analogue devices with adjustable potentiometers, analogue current outputs and alarm relays into smart, powerful, two-way digital transmitters with advanced features, such as automatic calibration and self-diagnostics, that can transmit more information, more accurately. This article will discuss advances in process analytical devices and how they can be applied to both new and existing pharmaceutical facilities.

Microbioreactor Array System Boost Cell Culture Capacity

Teva Absorbs Ivax In $7.4 Billion Bid

Protein Refolding Technology Improves Yields

Aptuit To Purchase Quintiles