
There are over 250 operations in the EU in various stages of development involving tissue engineering, regeneration and subsequent attempts at commercialization.

There are over 250 operations in the EU in various stages of development involving tissue engineering, regeneration and subsequent attempts at commercialization.
The type of robot used for placing and stacking the BFS cards is important. Conventional multi-axes designs have limited flexibility, often combined with high inertia that limits operating speeds.

Though dissolution testing has been under scrutiny, it is still a powerful test method.
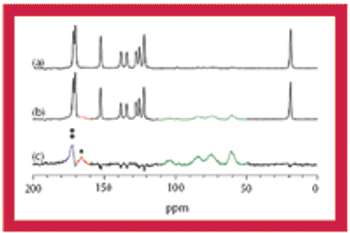
This article discusses the advantages and disadvantages of using solid-state NMR spectroscopy for the analysis of pharmaceutical solids.

Pfizer Combats Counterfeiters with RFID

Sanofi Pasteur and BD Team Up to Produce Microneedle Vaccines
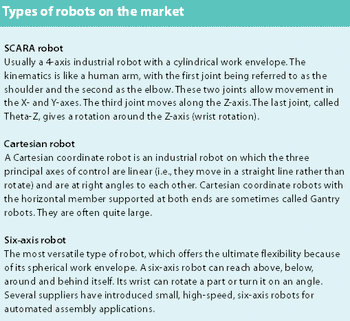
There is a rapidly growing ageing population that requires sophisticated medical devices and newer drugs. This is likely to result in an increase in the use of robotics to improve manufacturing efficiency. This article looks at the role of SCARA robots in pharmaceutical plants and laboratories.
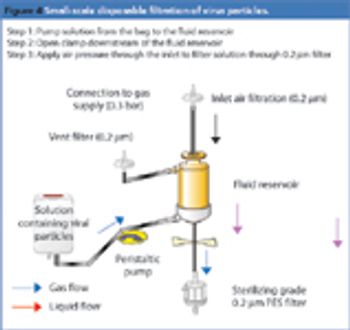
The medical industry was the first to understand the benefits of using disposable devices, such as needles and syringes, to prevent risks of cross contamination. The technology was then extended to blood transfusion activities, and it was only 10–15 years ago that the biopharmaceutical industry started to use disposables. Initially, most of the applications were limited to storage, involving bags, tubing and filter capsules. Since then, significant progress has been made in the polymer and plastics industry; in particular, a number of organic polymers have been developed that are resistant to gamma irradiation, autoclaving and even sterilization-in-place, rendering the technology attractive and usable by the biopharmaceutical industry. Now, the industry is moving beyond storage-focused disposable technologies to more complex processing applications.
FDA has tightened up the approval of new drugs, ensuring careful scrutiny of clinical data and stringent scanning to analyse the probable side-effects of a new drug

The closed vial has been developed to improve aseptic filling quality and to reduce process complexity. A ready-to-fill closed vial consists of a sterile vial provided with the stopper secured in place. The vial is filled by inserting a non-coring needle through the stopper, which is then resealed by laser.

Is the lack of finance necessarily spelling doom for the European biotech industry?

The big question for pharmaceutical services providers coming into 2006 is: will the good times continue to roll?

Manufacturers, FDA, and research organizations are collaborating on efforts to spur innovation and streamline drug production.
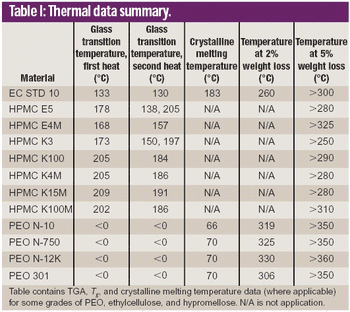
Hot melt extrusion (HME) formulation development depends heavily on choosing the appropriate polymers. This article reviews HME process parameters and highlights three polymers in HME: polyethylene oxide, ethylcellulose, and hypromellose.

Contract manufactures are faced with multiple challenges when determining whether to implement process analytical technology into their clients' or their own infrastructure.

Amgen Losing Hold on Anemia Drug Market

Bristol-Myers Squibb to Cut Costs by $500 Million

Roche Selects 12 Potential Manufacturing Partners for Tamiflu

FDA issues a warning letter to Nephron Pharmaceuticals.

SEC Loosens Revenue-Recognition Rules for Vaccine Stockpile Participants

NIAID Boosts Vaccine Innovation but Draws Controversy

The monopoly held by the large pharmaceutical companies within drug discovery could be falling into the hands of biotechnology firms claims Frost and Sullivan.
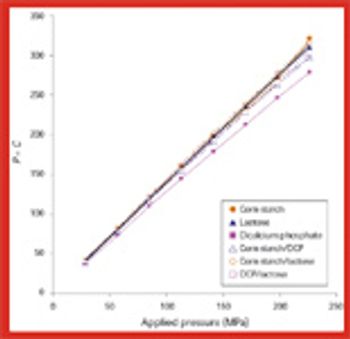
The survival of Bacillus subtilis spores in dicalcium phosphate, lactose, and corn starch and in their binary mixtures depends on the compressional properties of these materials and on parameters involved during the tableting process, including compression speed.

From politics to paychecks and downsizings to deadlines-what it's like to work for one of the world's largest industries.

If judges and juries lack the scientific knowledge to decide drugsafety cases, how can we protect both companies and patients?