
Respondents cited instrument maintenance and downtime, complexity of testing requirements, and time-consuming sample preparation as the top challenges in their laboratories.

Respondents cited instrument maintenance and downtime, complexity of testing requirements, and time-consuming sample preparation as the top challenges in their laboratories.

Pfizer’s new format of ibuprofen (AdvilR Liqui-GelsR Minis) uses Catalent’s softgel technology to deliver the formulation in a more concentrated fill, resulting in smaller capsules.

GE Healthcare’s Dharmacon business and CordenPharma contract manufacturing enter a strategic collaboration to accelerate the oligonucleotide development process.

Recro Gainsville expands its tableting capacity with the addition of a tablet press and film coater.

The suite installation increases PCI’s Hay-on-Wye site’s serialization capability to support clients in advance of meeting the implementation dates of the European Falsified Medicines Directive.

A $5.5 billion acquisition brings Capsugel’s oral dosage delivery capabilities to Lonza’s portfolio.

Samsung BioLogics signs $55.5 million agreement to manufacture tildrakizumab for Sun Pharma.

Patheon will add spray drying, sterile manufacturing, and packaging capabilities to four facilities.

How has the bio/pharmaceutical contract manufacturing industry evolved over the years and what does the future hold?

As pharma models changed during the past 40 years, contract manufacturing capacity and services evolved to meet demand.

The more pharma science and technology change, the more business and policy concerns stay the same.

SK Biotek plans to operate the Swords, Ireland API manufacturing facility as a contract development and manufacturing site.

Sartorius Stedim Biotech combined the company’s ambr 15 bioreactor system with the Nova BioProfile FLEX2 cell culture analyzer for laboratory experiments.
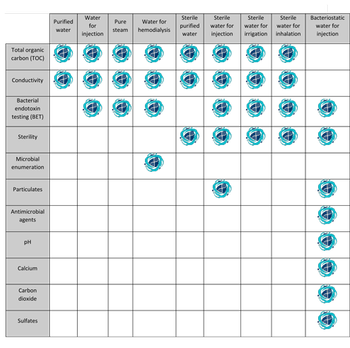
USP describes analytical and microbiology testing required for different types of pharmaceutical water.

Cambrex announces expansions of its North Carolina API pilot plant and Kalskoga, Sweden large-scale manufacturing facility.

Increased capacity at PPD’s Richmond, VA laboratory are designed to support customers’ vaccine development programs.

Expanded storage capacity at the SGS laboratory in Mississauga, Canada increases stability testing capabilities.
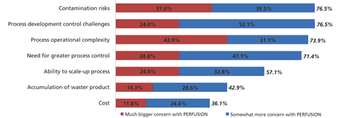
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.

Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

BioVectra will open its new microbial fermentation and complex chemistry site, including the capability to handle high-potency APIs, by the end of 2017.

The company expanded its UK storage facility to meet increasing demand from its growing customer base.

The company installed the FlexFactory to support its biosimilar production.

The compendium provides a resource to identify and locate targeted suitable patient-derived xenografts based on specific histology and molecular properties.

The new storage and distribution facility provides PCI with additional space, complementing its existing footprint currently used for specialist clinical-trial logistics as well as packaging, labeling, and qualified person activities for investigational medicinal products.

Catalent has signed an agreement with Therachon to support preclinical and clinical development of TA-46, a novel protein being developed to treat achondroplasia.