
Changing demands in drug development lead to new service combinations and models.

Changing demands in drug development lead to new service combinations and models.

When embarking on a new outsourcing project, there are a few initial steps that must be taken to ensure a project runs smoothly from the start.
Bristol-Myers Squibb's fit-for-purpose mode for clinical-trial materials for early-stage development seeks to achieve a better way in resource allocation. This article is part of a special issue on API Development, Formulation, Synthesis and Manufacturing.

The current trend in the pharmaceutical industry for the manufacture of small-molecule therapeutic agents is moving toward continuous flow processes. This article is part of a special issue on APIs.

In an increasingly competitive landscape, outsourcing providers are under mounting pressure to get their name out there and secure new and repeat business.

The author presents recent developments in simulated moving-bed chromatography in production of active pharmaceutical ingredients and intermediates. This article is part of a special issue on APIs.
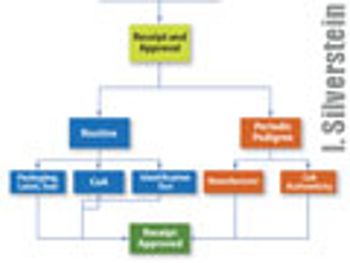
The author explains the background behind the excipient pedigree and how to implement its use.

The economic crisis has affected outsourcing providers in several ways.

India has evolved from a low-cost region to an area with extensive technical capability and high quality standards.

Every biopharmaceutical is unique and products are defined as much by their manufacturing process as their analytical characterisation. Because of this, the development of biologics has many inherent complexities over small molecule projects.

The main advantage of outsourcing highly potent API (HPAPI) manufacture is that it eliminates the need to invest in expensive containment infrastructure, which can also be complex to engineer, install and maintain.

When assessing the competencies of an outsourcing service provider, sponsor companies must pay a great deal of attention to the cGMP compliance level of the provider.

How to find the right partner in Asia–Pacific is a very complex question and there is no uniform answer.

Outsourcing early drug development can be viewed from a tactical or strategic perspective. These perspectives are often driven by the size of the company and provide different advantages.

Contract manufacturers must view their relationships with clients as a partnership, which lasts beyond the fulfillment of a particular project, and not simply as a single transaction.

Week of Aug. 23, 2010: Company and People Notes: Quark and Novartis Form Agreement; Hospira CEO Retires; And More.

Eli Lilly Ends Semagacestat Program; Selecta Appoints CEO; And More.

Sen. Michael Bennet (D-CO) introduced a bill earlier this month, the Drug Safety and Accountability Act of 2010, which is designed to improve regulatory oversight of drug-manufacturing facilities and improve related quality standards and monitoring.

Penwest and Endo to Merge; Watson Makes Senior Appointments; And More.

FDA Approves Flu Vaccines; Caraco Names COO; And More.

Fallout escalates from McNeil recall and Genzyme shortages as regulators review oversight.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

Manufacturers and regulators on both sides of the ocean move to ensure the safety of heparin and other globally distributed drug products.

Industry can meet its responsibility to society by considering innovative pricing and partnerships.

Relationship management is a limiting factor to growth in biomanufacturing outsourcing.