
The increased trend of outsourcing coupled with a relatively strong economy has seen the fine chemicals market grow at a very high level when compared to historical data.

The increased trend of outsourcing coupled with a relatively strong economy has seen the fine chemicals market grow at a very high level when compared to historical data.

As contract manufacturers and fine-chemical suppliers gather for CPhI/ICSE, effective strategies for technology differentiation are key in an increasingly competitive environment.

A Q&A with Brian Johnson, senior director of supply chain security at Pfizer, moderated by Patricia Van Arnum. Part of a special Ingredients issue.

Last week, Ben Venue Laboratories decided to exit the contract-manufacturing business during the next several years, thus ending more than 70 years of service in this field. To ensure the supply of medically necessary products, the company will work with its customers to develop and execute long-term transition plans.
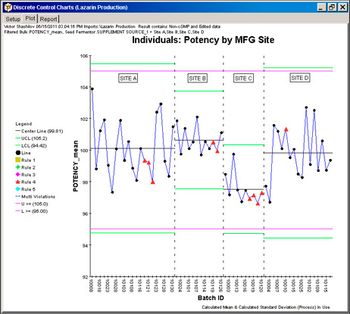
Using information technology tools to enhance process understanding helps reduce variability that can affect speed to market.

The Society for Chemical Manufacturers & Affiliates commented on EPA's modifications to the Inventory Update Reporting rule, also broadly know as the Chemical Data Reporting rule. EPA issued the final rule earlier this month.

The International Society for Pharmaceutical Engineering published a guidance document that defines current best practices in pharmaceutical manufacturing applications for handling gases that come into direct contact with the biopharmaceutical and pharmaceutical process steams.

Rising imports and overseas production spur realignment of enforcement.

Contract manufacturing organizations throughout Asia are increasing their capabilities to meet market demand and attract foreign investment and partnerships.
China rises to the top as a destination for international outsourcing.

Visiting a new site or going down memory lane may not get you where you want to go.

Terry Novak, president of Norwich Pharmaceuticals, on recent industry trends.

Quality management requires more effort in a complex supply chain.

Thomas P. Layloff describes the advantages of using thin-layer chromatography methods for counterfeit detection. This article contains bonus online material.

Analytical detection techniques help combat counterfeit drugs.

Respondents to the 2011 PharmSource-Pharmaceutical Technology Outsourcing Survey paint a positive picture, but concerns linger under the surface. This article is part of a special issue on Outsourcing.

Manufacturers are facing ever-increasing competition while end users are expecting better value and efficacy. The authors discuss these challenges and offer practical solutions.
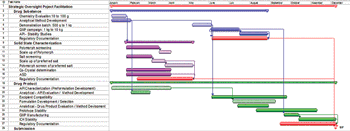
The article examines a cross-functional supplier integration model to facilitate project management.

The authors review current industry challenges and trends in managing global supply chains and propose best practices for improving visibility into those networks.

Pfizer's Experience with QbD. This article is part of a special issue on Outsourcing.

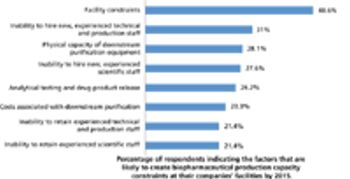
Supplier differentiation is increasingly important in the highly competitive arena of pharmaceutical outsourcing.
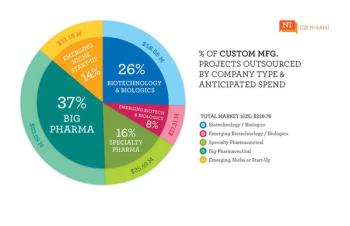
The article examines the drivers of customer perception of contract service providers of pharmaceutical development and manufacturing.

The authors share their approach and experience working in complex, multicompany environments for in-licensed products to develop successful chemistry, manufacturing, and controls packages for managing outsourcing partnerships.

An Industry Roundtable Moderated by Patricia Van Arnum and Rich Whitworh. Contract service and technology providers share their perspectives on the influence of quality by design in the expectations between sponsor companies and outsourcing providers.

Avantor reviews its experience with the Rx-360 shared audit pilot program, which is aimed at protecting the pharmaceutical supply chain. This article is part of a special issue on Outsourcing.