
Brazil develops its first national plasma fractionation plant.

Brazil develops its first national plasma fractionation plant.

A single, global tooling standard would offer many benefits, but one has been slow to emerge.

Q&A with Robert Hardy, chief executive of Aesica
The author provides an overview of QbD implmentation for biopharmaceuticals.

The power of emerging markets is reflected in the pharma's sales and production positions.

As drug shortages make headlines, FDA tests the Sentinel safety system and its efect on healthcare.

INTERPHEX 2011 aims to address the industry's unique characteristics.

Gilead to Acquire Calistoga; Bayer Healthcare Appoints Former Pfizer Exec; and More.

Company and People Notes: sanofi to Acquire Genzyme; Lilly Makes Senior Appointments; and More.

US Senators Amy Klobuchar (D-MN) and Bob Casey (D-PA) introduced the "Preserving Access to Life-Saving Medications Act," which is intended to help address and prevent shortages of prescription drug medications.

Pfizer Acquires Ferrosan's Consumer Health Business; Patheon Appoints Former Biogen Exec as CEO; and More.

Company and People Notes: Pfizer Completes King Tender Offer; Cook Pharmica Names VP of R&D and CSO; and More

Follow-ons were all the rage at this year's JP Morgan Healthcare Conference.
Will 2011 be a more promising year for new molecular entities? A review of Big Pharma's late-stage pipeline shows what might lie ahead.

As biologic-drug patents move toward expiration in the US, Indian firms with experience in the follow-on biologics arena are eager to partner with global manufacturers and secure their place in the growing biosimilars market.

Legislation has hampered cross-border drug importation and limited choice.

Q&A with Magnetrol International's Dan Klees

Food-safety, transparency, and counterfeit-drug growth will tax agency resources.
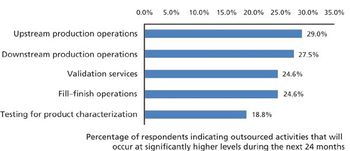
More crucial biomanufacturing operations are expected to be outsourced.

Anticounterfeiting solutions for vials and syringes.

As the industry prepares for Informex, the trade show of custom and batch manufacturers in Charlotte, North Carolina, a roundup of key recent developments.

Innovations protect the quality of temperature-sensitive products.

The authors highlight the need for a technology-transfer process that is efficient, cost-effective, and repeatable, stressing the importance of process understanding. Read this and other preferred organization articles in this special issue.

The article examines pharmaceutical market growth, company positioning, and the innovation potential in emerging markets. Read this and other preferred organization articles in this special issue.

A contract-service provider roundtable, featuring Albemarle, Baxter, DPT, Pfizer CentreSource, Dr. Reddy's, SAFC, and Vetter. Read this and other preferred organization articles in this special issue.