
When validating automated systems from third-party providers, using the V model and failure modes effects and criticality analysis (FMECA) early in the process can help.

When validating automated systems from third-party providers, using the V model and failure modes effects and criticality analysis (FMECA) early in the process can help.

The FDA has said it would like to amend postmarket safety reporting regulations ?to require that manufacturers and other facilities subject to current reporting requirements submit their reports in an electronic format".

The US Food and Drug Administration announced in an Aug. 20, 2009 release that it would like to amend postmarket safety reporting regulations "to require that manufacturers and other facilities subject to current reporting requirements submit their reports in an electronic format."

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Also, USP signed a Memorandum of Understanding with the Permanent Commission of the Pharmacopeia of the United Mexican States, more...

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

The US Food and Drug Administration announced a new program regarding the issuance of warning letters to the pharmaceutical industry.

The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.

Uncovering the root cause of contamination and leveraging the collaborative learning loop of QbD -Aegis Whitepaper

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

US Food and Drug Administration Commissioner Margaret Hamburg outlined last week six initial steps designed to improve the effectiveness and timeliness of FDA's regulatory and enforcement system.

On August 6, the Biotechnology Industry Organization (BIO) filed an amicus brief asking the US Supreme Court to overturn Bilski v. Doll, a decision of the US Court of Appeals for the Federal Circuit.

The US Food and Drug Administration released its Guidance for Industry titled Pharmaceutical Components at Risk for Melamine Contamination.

Also, EMEA gives public access to GMP data; SOCMA speaks out on toxic substances; more...

The Society of Chemical Manufacturers and Affiliates (SOCMA) reported in late July that Eurostat, the Statistical Office of the European Communities, recently published a baseline study or the first snapshot of REACH policy in the preregistration phase.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...

The Energy and Commerce Committee of the US House of Representatives approved H.R. 3200, America's Affordable Health Choices Act, last Friday.

The US Food and Drug Administration and European Medicines Agency (EMEA) launched this week a joint initiative to collaborate on international good clinical practice (GCP) inspection activities.

South Africa was the first country on the African continent to become a PIC/S member. The country's Director of Inspectorate and Law Enforcement describes the 10-year process.

Biotech firms must first close the gaps between science and biology on the path toward QbD.

The author of an ambitious book about quality control falls short of reaching his goals.
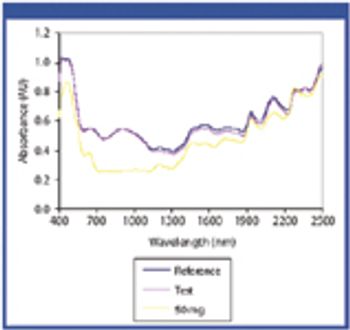
The authors applied near-infrared (NIR) spectrophotometry to assess whether eight drug products were authentic or counterfeit.

Individuals and companies at the top seem to have no problem short-circuiting their success.

Pressure to reduce healthcare spending has put drug rebates, price cuts, and tax hikes on the table.

An ounce of contamination usually leads to a mountain of investigation.