
Government funding is slated to boost comparative studies of prescription drugs.

Government funding is slated to boost comparative studies of prescription drugs.

Products at INTERPHEX focused on protection, compliance, and deterring counterfeiting. This article contains bonus online-exclusive material.

The organizations' presidents discuss market exclusivity, approval processes, and pending legislation.

Technology can solve enterprise-level problems.

With a new head of the FDA expected to be announced imminently, the pharmaceutical industry waits to witness the changes that will inevitably accompany the new appointment. These changes could, however, also impact the rest of the world's pharmaceutical markets.
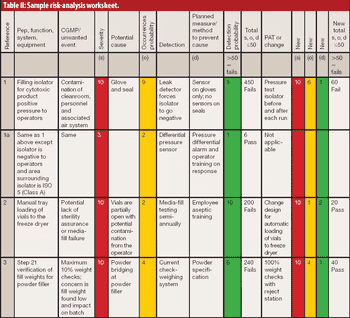
The author reviews the draft guidance on process validation, its QbD applications, and its potential impact on sterile manufacturing operations.

The author provides a history of the information chapter USP ‹1211› "Sterilization and Sterility Assurance of Compendial Articles," from the early 1900s to the current version.
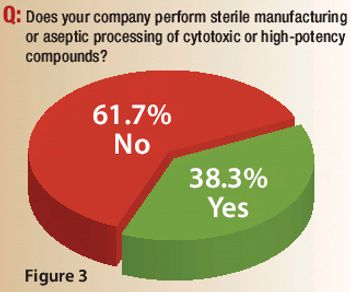
A Pharmaceutical Technology survey examines capacity expansions, outsourcing practices, innovation levels, and the role of quality by design in sterile manufacturing and aseptic processing.

The author describes various manufacturing processes and evaluates whether the guidance can be applied to each of them.

Senators Chuck Grassley (R-IA) and Ted Kennedy (D-MA) introduced legislation last Thursday that would give the US Food and Drug Administration more resources to inspect domestic and foreign plants that manufacture drugs and medical devices.

The Pharmaceutical Research and Manufacturers of America released a statement this week in response to recent media reports regarding the amount of pharmaceutical ingredients being discharged by manufacturing facilities into the environment.

The US Food and Drug Administration has agreed to address some core industry questions regarding the changes made to US Pharmacopeia General Chapter <467> in July 2008.

The US Food and Drug Administration finalized a Guidance for Industry this week that aims to clarify the submission of new drug applications (NDAs) and biologics license applicants (BLAs) using the common technical document (CTD) format, including the electronic CTD (eCTD).

The US Food and Drug Administration issued a draft guidance for industry on the Submission of Summary Bioequivalence Data for Abbreviated New Drug Applications (ANDAs).

Also: Sanofi-aventis acquires BiPar Sciences; FDA issues Warning Letter to Chinese heparin manufacturer; Halo Pharmaceutical appoints chief scientific officer; more...

On April 2, the Health Products and Food Branch of Health Canada and EU (consisting of the European Commission [EC] and European Medicines Agency [EMEA]) released "Implementation Plan for Regulatory Cooperation on Medicinal Products."

The United States Pharmacopeial (USP) Convention signed a memorandum of understanding (MOU) with the Federal Service on Surveillance in Healthcare and Social Development of the Russian Federation (Roszdravnadzor) last week in Moscow.

In late March, the US Food and Drug Administration sent warning letters to nine companies to stop manufacturing 14 unapproved narcotic drugs. Less than two weeks later, on Apr. 9, the agency amended those letters when it realized that one particular unapproved opioid (a high concentrate of morphine sulfate oral solution) is desperately needed by patients.

ATSM and ICH approaches, in place of traditional qualification or integrated commissioning and qualification (C&Q) using impact assessment, can make projects and processes more efficient and help facility owners and designers ensure compliance, quality, and safety when defining acceptance criteria for their critical process systems and equipment.

The US Food and Drug Administration issued 14 untitled letters last week to pharmaceutical companies in violation of the Federal, Food, Drug, and Cosmetic Act for publishing misleading and misbranded information about their drug products online.

Although industry is tightening its belt, contract manufacturers across Europe are actually making out quite well by taking on additional projects and new roles.

Senator Charles Grassley (R-IA) sent a letter to Frank Torti, acting commissioner of the US Food and Drug Administration, to express concern about a memo that Torti sent to agency staff.

Also, SOCMA changes name; two FDA approvals; Biogen Idec names chief operating officer; more...

The United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) seized nearly half a million pounds worth of counterfeit medicines on Mar. 26, 2009 in Middlesbrough, England, according to a MHRA press release.

Brief pharmaceutical news items for April 2009.