
The companies are collaborating on the commercialization of two biosimilar candidates in the US and Canada.

The companies are collaborating on the commercialization of two biosimilar candidates in the US and Canada.

A report from the European Union Intellectual Property Office shows that the EU loses approximately €10.2 billion a year due to counterfeit medicines.

The Intelligent Control Inhaler is an intuitive, fully-integrated device delivering accurate doses of medication to patients, while providing on-screen instructions for use and feedback to the patient and healthcare provider via an app.

The time and resources required to finalize post-approval changes may be preventing manufacturers from modernizing facilities, or even scouting for new technology.

The company announced that it will build a new manufacturing facility at the GMR Aerospace Park at the Rajiv Gandhi International Airport in India.

Constantia Flexibles acquired a flexible packaging business from Lamp San Prospero SPA.

Value assessment initiatives are expected to have a major impact on drug use and reimbursement.

Xellia added laboratory space and personnel in Zagreb, Croatia to work on anti-infective products that combat the antimicrobial resistance problem.

Tosoh Bioscience adds TOYOPEARL Sulfate-650F, a strong cation exchange resin that exhibits high salt tolerance, to its process media product line.

Bio-Rad Laboratories’ Nuvia IMAC Resin, an immobilized metal affinity chromatography resin, is optimized for use from laboratory-scale through pilot studies to process-scale manufacturing.

The Phase III trial examined guselkumab compared with a placebo and Humira at treating moderate-to-severe plaque psoriasis.

Allergan entered into a licensing agreement with AstraZeneca for MEDI2070, an anti-IL-23 monoclonal antibody in phase IIB development for the treatment of patients with moderate-to-severe Crohn’s disease.

The company invested in a new cGMP facility located in Berlin, Germany.

The new company will focus on R&D of cancer treatments.

The hospital received a five-year $5 million grant from CDC to survey for communicable diseases in children and evaluate vaccine effectiveness.

ViraTherapeutics will develop its VSV-GP therapy alone and in combination with other therapies.

FDA’s Center for Drug Evaluation and Research provides an update on its safety initiatives.

he guidance addresses the good manufacturing practice for managing quality in APIs.

Pharmaceutical manufacturers and business partners are studying how Advanced Digital Ledger Technology might solve supply chain and other data transfer problems.

The company is voluntarily recalling all unexpired lots of 50-mm, 0.2-micron filters.

EDQM and the Japanese Pharmacopoeia will improve the sharing of information on therapeutic products that are common in both Europe and Japan.

PaizaBio will add aseptic injectable production capacity in Hangzhou, China.
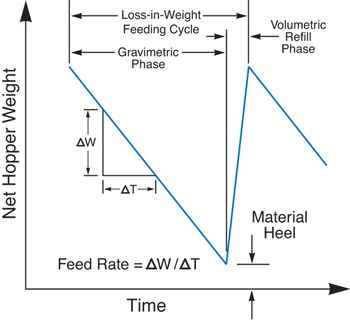
Designing loss-in-weight feeders for accurate and consistent refill is crucial to a continuous solid-dosage process.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable drugs.

The editors seek drug development experts to contribute technical articles for 2017.

Tapemark, a full-service CDMO specializing in transdermal, oral thin film, and topical drug-delivery technologies, is exhibiting at CPhI.

Could grading finished-drug manufacturers on their quality and efficiency lead to greater transparency, improve quality and reduce drug shortages?

The agency appealed two interim orders to suspend the release of documents requested by third parties.

Ravi Limaye gives an overview of the biosimilar industry and projects for 2020.

Gil Roth gives an overview and outlook for the CDMO industry in 2016.