
Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...

Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...
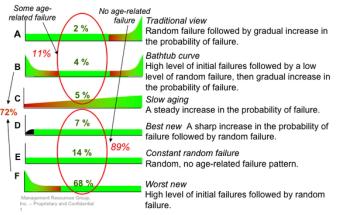
Time-based maintenance programs preserve the equipment but usually not its function, and they do not mitigate equipment failure for the balance of a machine's life cycle.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the February 2009 edition from PortaFab and Sterling.

The spotlight on the biopharmaceutical industry is intensifying, as recently evidenced by Pfizer's (New York) ongoing acquisition of Wyeth (Madison, NJ), which was initiated partly to reduce the former's dependence on small-molecule drugs.

Also, Penn Pharma to expand; stem cell research funding ban lifted; Bristol-Myers Squibb made senior appointments; more...

Merck & Co. (Whitehouse Station, NJ) and Schering-Plough (Kenilworth, NJ) completed a definitive merger agreement under which Schering-Plough stockholders will receive $23.61 per share.

Ending a long, closely watched debate over the issue of federal preemption, the US Supreme Court on March 4, to uphold a $6.8 million Vermont Supreme Court decision of Diana Levine against Wyeth Pharmaceuticals (Madison, NJ).

The Synthetic Organic Chemical Manufacturers Association (SOCMA) said last week that Congress is likely to the include inherently safe technology (IST) measures in proposed chemical site-security legislation that is likely to be introduced in late winter or early spring.

Senators Byron Dorgan (D-ND), Olympia Snow (R-ME), John McCain (R-AZ), and Debbit Stabenow (D-MI) introduced a bill March 4 that would allow pharmacists and wholesalers to import prescription drugs from Australia, New Zealand, Japan, Switzerland and the European Union.

Weakening fundamentals in 2008 and projected for 2009 dampen the outlook for pharmaceutical chemical outsourcing, but near-term growth and investments levels in R&D and capital spending remain fairly robust.

Gilles Cottier, SAFC's new president, plans to continue a growth strategy that emphasizes value-added technologies positions, growth in Asia, and customization in its supply-chain solutions.

President Obama nominated Kansas Governor Kathleen Sebelius as his new Secretary of Health and Human Services (HHS).

President Obama's Fiscal Year 2010 (FY 2010) budget includes support for a regulatory pathway for follow-on biologics.

On March 3, the US Food and Drug Administration released a draft guidance for Industry entitled "Clinical Pharmacology Section of Labeling for Human Prescription Drug and Biological Products: Content and Format."

In a press release dated Feb. 25, 2009, the US Food and Drug Administration charged that Ranbaxy Laboratories's (Gurgaon, Haryana, India) Paonta Sahib facility falsified data and test results in approved and pending drug applications.

FDA issued a notice in the Federal Register last week requesting comments on the automated collection of four additional pieces of information that are not available in the US Customs and Border Protection's (CBP) data set.

Also, Schering-Plough's vaccine unit, Nobilon, formed an agreement with the World Health Organization; Ore Pharmaceuticals named president and CEO; more...

Scientists studying epilepsy have traditionally focused on the comings and goings of ions through molecular channels in nerve cells, and many current antiseizure therapies seek to modulate that dynamic.

USP's Stage 2 heparin monograph revisions address identification, potency, and impurities.

USP's revised chapter on injectables could harm anticounterfeiting efforts and drug administration.

The source of a problem reveals itself after some investigation, or it may crash down on you.

An updated book provides essential information for scientists who monitor microbial quality.

With the economy down and multinational firms capitalizing on patents in developing countries, India's R&D sector still has a long way to grow.

This month's editor's comment.

Earlier this week, House Appropriations Committee Chairman Rep. David Obey (D-WI) introduced the FY 2009 Omnibus Appropriations Act (H.R. 1105).

Also, GPC Biotech AG and Agennix to merge; BASi appoints COO of scientific services; more...

The United States Trade Representative (USTR) is seeking documentation from chemical companies to identify possible non-tariff trade barriers, created by the European Union's Registration, Evaluation, Authorization and restriction of Chemicals (REACH) regulation, which would be inconsistent with the EU international trade obligations under World Trade Organization (WTO) rules, according to an informational release by the Synthetic Organic Chemical Manufacturers Association (SOCMA).

The US Food and Drug Administration stated its considerations in regards to the three draft annexes to the International Conference on Harmonization's Q4B document.

The US Food and Drug Administration has released a draft guidance for industry entitled Influenza: Developing Drugs for Treatment and/or Prophylaxis.

The US Food and Drug Administration posted the Prescription Drug User Fee Act (PDUFA) IV Drug Safety Five-Year Plan on its website.