
January was another very bleak month financially, but luckily it ended on a bit of a political high...

January was another very bleak month financially, but luckily it ended on a bit of a political high...

During the last 20 years, pharmaceutical R&D spending has increased 15-fold while new product approvals have increased only 0.70-fold.

The American Society for Quality (ASQ), a membership organization that focuses on quality training in several industries including pharmaceuticals and healthcare, has adopted a new certification for good manufacturing practice (GMP) professionals.

Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Senators Chuck Grassley (R-IA) and Herb Kohl (D-WI) have introduced S. 301, known as the Physician Payments Sunshine Act of 2009 to the 111th Congress.

Pfizer will purchase Wyeth (Madison, NJ) under a definitive merger agreement approved by both companies' boards of directors.

Also, Elan explores strategic alternatives; NanoGuardian appoints John D. Glover to lead its Security Advisory Board; more...

Eli Lilly and Company has reached resolution regarding its previously reported government investigation into the company's US marketing and promotional practices for "Zyrexa" (olanzapine), an antipsychotic drug, thereby ending a government investigation started in 2004.

Last week, the US Food and Drug Administration issued a draft guidance for industry, Standards for Securing the Drug Supply Chain–Standardized Numerical Identification for Prescription Drug Packages, and launched a pilot program to help protect the pharmaceutical supply chain.

The US Department of Health and Human Services's Biomedical Advanced Research and Development Authority has awarded Novartis a contract valued up to $486 million over eight years to support the design, construction, validation, and licensing of a US cell-based influenza vaccine manufacturing facility in Holly Springs, North Carolina.

The US Food and Drug Administration issued its Draft Guidance for Industry on Submission of Laboratory Packages by Accredited Laboratories.

One relatively new dosage form is the orally dissolving strip, a thin film formulated with hydrophilic polymers that rapidly dissolves on the tongue.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the January 2009 edition from Clark Solutions and Neopac.

An integrated information technology environment can help pharmaceutical companies find new processes and approaches to improve manufacturing efficiency, productivity, and quality.

A draft guidance for industry titled "Good Importer Practices" has been released to provide importers guidance on the steps they can take to help ensure imported products comply with the relevant statues and regulations of the United States throughout a product's lifecycle.

The US Food and Drug Administration announced that it had filed a Consent Decree on Dec. 23, 2008 and was awaiting the court?s entry of a permanent injunction that bars Actavis (Hafnarfjordur, Iceland), its officers Sigurdur Oli Olafsson and Douglas Boothe, and its subsidiary Actavis Totowa (Totowa, NJ) from manufacturing and distributing drugs at the Actavis Totowa facilities.

Frank Torti, currently principal deputy commissioner and chief scientist of the US Food and Drug Administration, will take over as FDA's acting commissioner next week, according to an official with the US Department of Health and Human Services (HHS).

Also, Bristol-Myers Squibb forms collaboration with ZymoGenetics, Hana Biosciences appoints VP of regulatory affairs, more...

A December report on healthcare from the Congressional Budget Office (CBO) proposes that an abbreviated regulatory process for follow-on biologics could result in more than $10 billion in savings by the year 2020.

Also, Novozymes Biologicals settles pollution case with the US Department of Justice; EntreMed restructures management team; more...

Michigan State University researchers have discovered a technique for viewing whole cells to gain an understanding of protein inclusion bodies.

The generic-drug manufacturer Actavis reached an agreement on a consent decree of permanent injunction with the US Food and Drug Administration regarding the company?s US subsidiary Actavis Totowa LLC.

A California jury ordered Pfizer (New York) to pay $38 million to the Ischemia Research and Education Foundation (IREF), a medical-research nonprofit group, for stealing trade secrets to develop its "Bextra" analgesic.

Outsourcing sterile manufacturing involves an integrated approach in product life-cycle management.

Achieving double-digit growth through 2011, biotech-based active pharmaceutical ingredients (APIs) are expected to far surpass growth rates for chemically synthesized APIs.

Although its domestic market is on the rise, Pakistan's market conditions have not proved welcoming enough to keep foreign investors.

The incoming administration has renewed hope for stem cells, but less adequate copycats may follow.

A book explains the many pharmaceutical applications of polymers from natural sources.
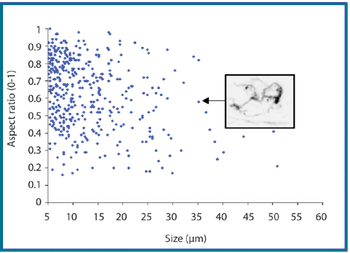
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.
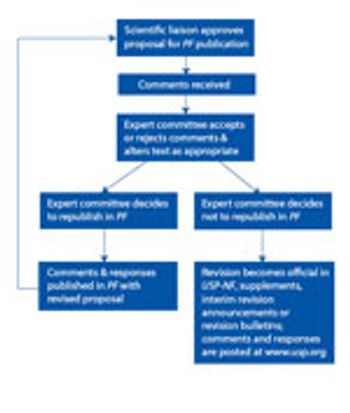
The USP public-comment process exists for a reason. Industry needs to take advantage. This article contains bonus online-exclusive material.