
Company and People Notes: Novartis Settles with US Attorney's Office; Hospira Names VP, And More.


Company and People Notes: Novartis Settles with US Attorney's Office; Hospira Names VP, And More.

The reopened debate over embryonic-stem-cell research could stifle many other scientific pursuits.

Editors' picks of pharmaceutical science and technology innovations.

The advantages of coupling hot melt extrusion technology with online FT-NIR spectroscopy are explained.
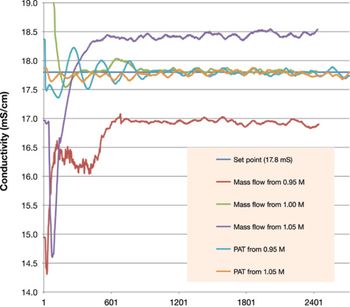
The authors describe the operational requirements and design of a process-ready PAT-based IBD system.

The author discusses new detection-system technologies that can improve performance and provides key criteria to consider when selecting or upgrading a system for pharmaceutical use.

Several industry experts describe applications in pharmaceutical applications, including on-line total organic carbon analysis, ultra-fast liquid chromatography, rapid microbial testing, and differential scanning calorimetry-Raman Spectroscopy.

The author outlines key considerations for carrying out a structured approach to monitoring process performance and ensuring product quality.

The author describes the development of small-angle X-ray scattering and analyzes its advantages in the characterization of drug-delivery systems and large molecules. This article is part of a special Analytical Technology issue.
The authors discuss the approach taken to develop a new calibration approach, its associated protocols, and how it can be used to calculate data.
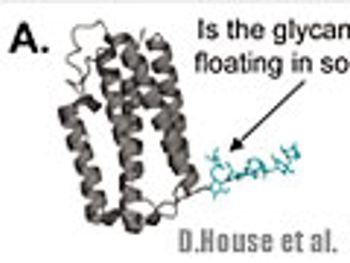
The authors review and discuss the influence of glycans on the conformation of a representative IgG1 biopharmceutical using H/DX-MS as an analytical tool.

Novartis Sells US Enablex Rights; Ricerca Names Chemistry Director; And More.

DSM and PolyTherics in Development Deal; Kite Pharma Appoints President and CEO, And More.

The US Food and Drug Administration recently issued a Warning Letter to Bristol-Myers Squibb (BMS, New York) for violations of current good manufacturing practice at the company's Manati, Puerto Rico, manufacturing facility.

Genzyme Sells Genetics Business; Bausch and Lomb Names Vice-President; and More.

US Pharmacopeia apparatuses for testing the dissolution of transdermal drugs produce good, reproducible results. Yet some scientists believe that further modifications could improve the instruments? suitability for this application.

Pfizer to Acquire FoldRx; Protalix Appoints COO; And More.

The Agilent 2100 Bioanalyzer plus the Agilent High Sensitivity Protein 250 Kit automatically detects and quantifies protein impurities down to 0.05% — meeting ICH guideline Q3B(R2)!

Company and People Notes: Lonza Acquired Vivante; PPD Appoints VP of Quality Management; And More.

Software and online monitoring are helping the pharmaceutical industry improve its corrective and preventive action programs. This article contains bonus online material.

To properly inspect based on measurement, a reference standard is crucial for comparison.

New data provide insight into pharma-industry professionals' daily lives.
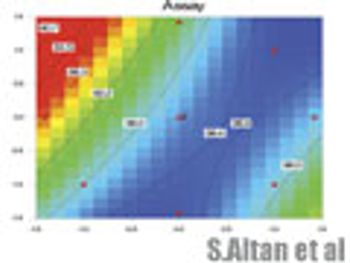
In the final article of a three-part series, the authors discuss how to present a design space and evaluate its graphical representation.

The authors discuss the capabilities of immobilization technologies and the ability to use an expanded range of solvents for mobile-phase components and solvent dissolutions. This article is part of a special issue on APIs.

Utilizing integrated active radio frequency identification (RFID) and real-time asset management systems can yield commercial and compliance benefits.