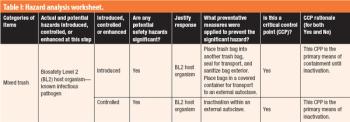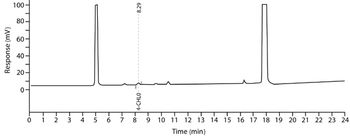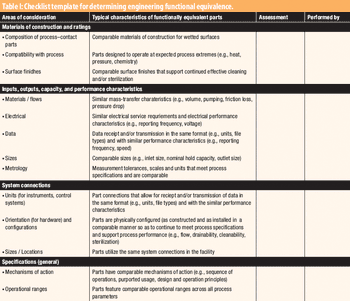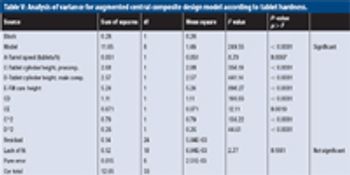
The author prepared and analyzed a detailed design of experiments for the manufacture of a simple tablet formulation. The aim was to test whether tablet hardness and weight could be controlled during the compression process by adjusting certain machine parameters.