
The BIO convention, and healthcare reform, could re-energize biotech.

The BIO convention, and healthcare reform, could re-energize biotech.

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and types of spending made in 2009 and planned for 2010.

Could insect cells offer a faster way of manufacturing pandemic influenza vaccines compared with traditional egg-based methods? According to researchers at the Vienna Institute of BioTechnology (Austria), their new technique could help a virus-like particle (VLP) vaccine to reach the market within 3 months from the first isolation of a new influenza strain - traditionally produced vaccines take approximately 6 months.

Vibrational spectroscopy, which encompasses near-infrared, mid-infrared and Raman spectroscopy, is an important analytical tool in the pharma industry.

Biotechnology company Genzyme reported that FDA notified the company that it intends to take enforcement action to ensure that products are made in compliance with good manufacturing practice (GMP) regulations.

Pfizer Invests In Nodality; SOCMA Approves New Members; And More.

Merck Ends Partnership With Dynavax; PhRMA Elects Officers; And More.

Abbott Agrees to Acquire Facet Biotech; FDA Issues Black-Box Warning For Plavix; and More.

The World Health Organization released new guidelines this week for the treatment of malaria and the first-ever guidelines on procuring safe and efficacious antimalarial drugs.

Exelixis And XenoPort Announce Job Cuts; GSK Dedicates India Facility; And More.

The US Food and Drug Administration recently published guidance for the characterization and qualification of cell substrates, viral seeds, and other biological materials used to manufacture viral vaccines for human use.

Astellas Pharma Makes Bid for OSI Pharmaceuticals; Ringo Retiring From Pfizer; And More.
Editors' Picks of Pharmaceutical Science & Technology Innovations

Could President Obama's tax reform, which is targeted at reducing outsourcing, endanger India's contract-services industry?

Pre-Interphex 2010 Product Releases.
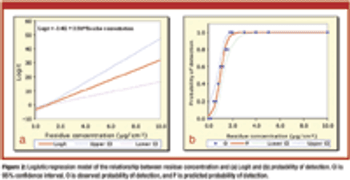
Current methods for establishing visible residue limits (VRLs) are not statistically justifiable. The author proposes a method for estimating VRLs based on logistic regression.

To best carry out the vision of Hatch-Waxman, Congress must act now on biogenerics.

Brief pharmaceutical news items for March 2010.

From last-minute product inserts to putting out fires, close calls are a common occurrence.

If both sides of the aisle don't agree on even mild healthcare reform soon, the bill could die out.

The vice-president addresses shifts in process and more.


The author reviews the benefits, challenges, and considerations to be made when selecting a gamma-irradiation sterilization method.
The authors describe an industrialized process for the manufacture of iPSC-derived human cardiomyocytes. This article is part of a special issue on Bioprocessing and Sterile Manufacturing.

Pharmaceutical Technology talked to drugmakers, equipment vendors, and service providers to learn more about the advantages and disadvantages of rapid microbial-detection methods.