
VaxGen Shareholders Reject OXiGENE Merger; Roche Creates Research Hub In Singapore; And More.

VaxGen Shareholders Reject OXiGENE Merger; Roche Creates Research Hub In Singapore; And More.

Cephalon Buys Mepha; BASi's CEO Retires; and More.
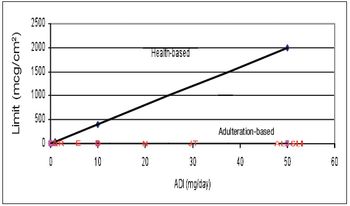
The author discusses how the use of a visible residue limit has made the 10-ppm cleaning limit obsolete in many applications.

Sharing too much-or too little-information can have disastrous onsequences.

FDA impersonators and counterfeit drugs threaten the public's trust in online pharmacies.
Editors' Picks of Pharmaceutical Science & Technology Innovations

A recent book provides information about formulating biopharmaceuticals that is easy to swallow.

Regulators and industry move to require inspections of API manufacturing facilities.

When it comes to healthcare rform, we must not overlook investment in innovative technologies.
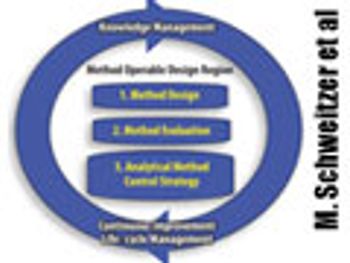
The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods. This article contain bonus online material.

Leading experts share insight on the current and future direction of process analytical technology. This article contains bonus online material.

Statistics are often viewed as confusing and complicated, but multivariate data analysis (MVA) methods can be used to amass knowledge simply.

Novartis CEO To Step Down; DSM To Close Netherlands Facility; And More.

FDA Issues Warning Letter To McNeil Healthcare; Charles River Laboratories Will Suspend Operations At Massachusetts Facility.

The United States Pharmacopeial Convention is recalling USP 33?NF 28.

Bioject Medical Technologies establishes alliance with MPI Research; Biogen Idec CEO to retire; And More.
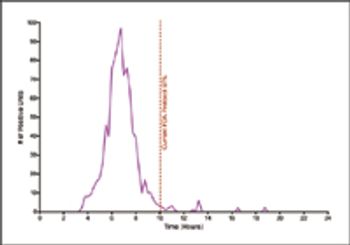
This study used biological indicators containing Geobacillus stearothermophilus spores and a new technology to continuously monitor incubated BIs and record nonsterile results.

A comprehensive book about mass transfer benefits from the author's personal touch.

Team leaders in FDA's Office of Generic Drugs provide an overview.

Plus, novel dosage forms and emerging trends.
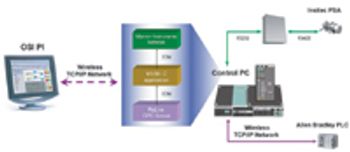
The authors describe the implementation of an on-line particle-size analyzer on an active pharmaceutical ingredient milling operation at a commercial site.
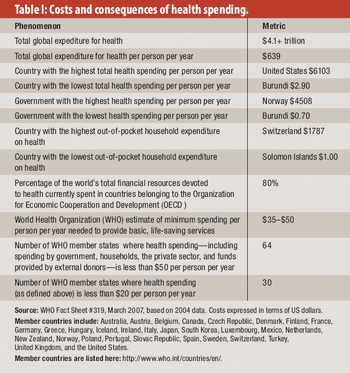
From healthcare to corruption to life expectancy, here's what we can learn from the past decade.

Directors and staff miss the mark when it comes to following procedures.

BioNanomatrix (Philadelphia) names Edward Erickson president and CEO; and More.

Merck & Co. Acquires Avecia Biologics; Ambrilia Biopharma Closes Manufacturing Facility; And More.