
Genzyme Sells Business Unit to Sekisui Chemical; Ablexis Names VP of Research; and More.

Genzyme Sells Business Unit to Sekisui Chemical; Ablexis Names VP of Research; and More.

The International Conference on Harmonization (ICH) Steering Committee (SC) and its working groups met in Fukuoka, Japan Nov. 6-11, 2010.

European confidence and optimism in biotechnology is increasing, according to a survey conducted by the European Commission.

The European Medicines Agency and the Massachusetts Institute of Technology's (MIT's) Center for Biomedical Innovation (CBI) and Center for International Studies (CIS) are launching a collaborative research project with a focus on enhancing regulatory science in pharmaceuticals, according to an EMA press release.

During the 20 months before the crisis of contaminated heparin in early 2008, the US Food and Drug Administration did not inspect any Chinese heparin firms, according to a US Government Accountability Office, (GAO) report.

Roche Details Restructuring Plan; Sigma-Aldrich Names Successors after CEO's Death; and More.

The biotechnology company Biogen Idec (Weston, MA) announced last week a major restructuring program that will refocus the company's research and development (R&D) programs, consolidate facilities, and reduce its workforce.

PQRI and FDA present Process Drift Workshop, and More.

The US Food and Drug Administration's Puerto Rico district office "may be having difficulty exercising oversight on the numerous pharmaceutical manufacturing facilities on the island," according to Rep. Edolphus Towns (D-NY), chair of the US House of Representatives Committee on Oversight and Government Reform.

Eli Lilly Acquires Avid Radiopharmaceuticals; EMA Recruiting New Director; and More

The market for orally disintegrating and fast dissolving tablets could exceed revenues of $13 billion by 2015 based on upward global growth trends, according to a report from Technology Catalysts International, a technology transfer and business consulting firm based in Virginia.

Sanofi-aventis (Paris) has asked Genzyme to stand aside and let the shareholders decide on whether an acquisition should take place.

The European Medicines Agency published a draft list of questions and answers on postapproval change-management protocols last week.

The growth in pharmaceutical outsourcing is creating a more complex and risky supply-chain environment, according to a report issued last week.

sanofi acquires BMP Sunstone; DCAT Names President; and More.

Pfizer (New York) announced on Oct. 29, 2010 that it intends to recall two additional lots--approximately 38,000 bottles--of Lipitor (atorvastatin calcium) 40 mg tablets distributed in the United States.

From fiscal year 2007 to 2009, the US Food and Drug Administration increased the number of foreign drug inspections it conducted, but the agency still conducted fewer foreign inspections than domestic inspections each year, according to a recent report by the US Government Accountability Office (GAO).

FDA Holds Biosimilars Public Hearing
Editors' picks of pharmaceutical science and technology innovations.

A recent book offers an excellent overview of cleaning validation.

A conversation with Mike de la Montaigne, president of Eisai Machinery, USA Inc., about the possibilities for conducting fully automated product inspections.

As technology advances, industry's needs are growing.
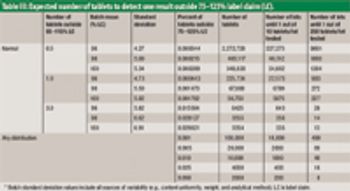
The authors describe a modified version of the Large-N test used to determine content uniformity.
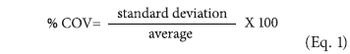
The author examines the process of method development, with reference to ISO 13320:2009 and relevant monographs from the United States and European pharmacopoeias.

Applied statisticians are forever searching for the enemy of quality-variability.