
The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.

The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.
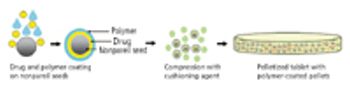
The current review describes the role and selection of excipients, pellet core, coating materials, and compression with various cushioning agents.

Two popular methods for detecting protein aggregates are analytical ultracentrifugation (AUC) and size-exclusion chromatography?multiangle light scattering (SEC?MALS). These techniques? results correlate relatively well, but each one has its own strengths.

Ensuring compliance though increased statistical knowledge and resources.
A holistic approach to establishing robust control measures.
Industry associations will soon provide new recommendations about extractables and leachables.

Recent recalls, including that of American Regent?s caffeine and sodium benzoate injection on May 5, 2011, highlight the importance of particulate inspection, and they might lead observers to ask whether current inspection methods are sufficiently effective.

How do you assign a minimum sample weight for a US Pharmacopeia <41> balance application when the tested repeatability gives a standard deviation of zero?
Editor's picks of analytical instrumentation products for May 2011.
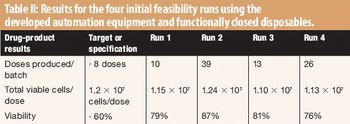
The authors developed automated equipment that uses functionally closed disposables to perform cellular and ribonucleic processing.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

Warner Chilcott announced in a press release on Apr. 18, 2011, its intentions restructure, placing 500 Western European jobs on the line.

Axcan acquires Mpex Pharmaceuticals; Bend Research receives patent for improving bioavailability of low-solubility drugs; and More.

The implementation of digital photomicrography has expanded the capabilities of microanalysis for quality control. But if used incorrectly, the technique can hurt more than help.

To find out how well industry is applying QbD, and what benefits the approach can bring, Equipment and Processing Report talked to Moheb M. Nasr, director of FDA?s Office of New Drug Quality Assessment.

FDA recently published guidance for preventing the cross-contamination of finished pharmaceuticals and active pharmaceutical ingredients with nonpenicillin beta-lactam antibiotics.

Editor's picks of analytical instrumentation products for April 2011.

The hardest errors to spot are the ones that don't look like errors at all.

Particle characteristics can affect pharmaceutical formulations and products in a number of ways, and a variety of techniques are available that enable particle monitoring and characterisation.

Many facilities buy compressed gas tanks or evaporate liquid nitrogen to supply processes with dry, high-purity nitrogen. An in-house nitrogen generator, however, provides several significant benefits.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the February 2011 edition from Emerson Process Management and Spirax Sarco.

At a conference on preserving national security at the University of Pittsburgh Medical Center last week, FDA Commissioner Margaret Hamburg stressed the importance of medical countermeasures for responding to natural and deliberate threats to public health.

The author presents a method to calculate the relationship between supply air volume flow and airborne particle concentrations.

The unit selected for wet granulation, whether it be a high shear mixer or a fluidised bed, has a marked impact on granule properties.

Pfizer Completes King Acquisition; ViiV Healthcare Names Chairman; and More.