
Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

Precedents set in the historic Barr case continue to raise questions over suitable sample-size criteria.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

The last in a series of eight case studies from the Product Quality Research Institute focuses on internal GMP audits.
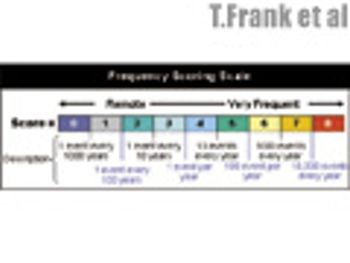
The sixth in a series of eight case studies from the Product Quality Research Institute focuses on packaging line GMP optimization.

On Sept. 27, 2011, FDA sent Genentech a Form 483 listing several violations at the company's South San Francisco, California, plant. The violations included problems with investigations into batch failures, inappropriate equipment design, and insufficient protection against contamination. FDA visited the plant, which produces the cancer drug Avastin, 13 times in September 2011 and made four observations.

Careful mixing during a product's distillation can help avert trouble from a strong concoction.
New product reviews for November 2011.
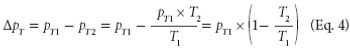
The authors describe the operational qualification of test accuracy with regard to temperature drift using a thermal-compensation algorithm on several freeze dryers.
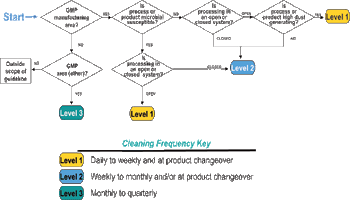
The fifth in a series of eight case studies from the Product Quality Research Institute focuses on nonsterile facility cleaning requirements.
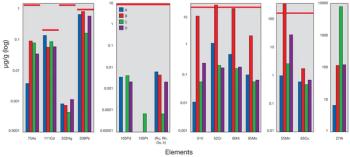
The US Pharmacopeia (USP) proposes to lower the maximum permissible limits of trace elements in pharmaceuticals and recommends that impurities be measured through automated instrumentation-based methods. The proposed regulations specify inductively coupled plasma–mass spectrometry (ICP–MS) and inductively coupled plasma–optical emission spectrometry (ICP–OES) as the techniques of choice. This article discusses the benefits of ICP–MS and ICP–OES for the accurate detection of trace elements in pharmaceutical products, in compliance with the proposed USP chapters.

The authors discuss the analytical methods and related testing for bioequivalence studies of ADCs.
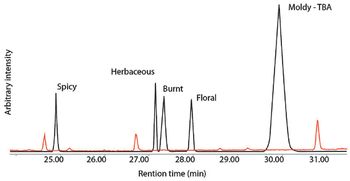
Combining olfactometry analysis with multidimensional gas chromatography–mass spectrometry provides an extremely useful analytical method for identifying aroma or odor notes from a sample.

A Technical Forum Moderated by Patricia Van Arnum, featuring contributions from PerkinElmer, BioTools, Chiral Technologies, Shimadzu Scientific Instruments, GE Analytical Instruments, and Waters.
This article focuses on the history of glass delamination and methods that detect it, both from a compendial and a research perspective.

The authors discuss the analysis of the resulting data, focusing on methods for the calculation of mass median aerodynamic diameter, one of the metrics routinely used for comparative testing.
The authors investigate the effect of low pH and ionic strength on aggregation using turbidity measurements and size-exclusion–high-performance liquid chromatography.
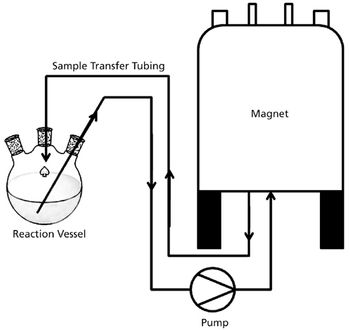
The authors describe the benefits of nuclear magnetic resonance (NMR) compared with traditional monitoring techniques. They also discuss how NMR reaction monitoring provides a new process analytical technology tool for industry.

Continuous manufacturing is increasingly noted as an important long-term objective for the pharmaceutical industry. PTE talks with Tim Freeman, Director of Operations at Freeman Technology, about some of the central issues involved in this transition, as well as the supporting role of relevant analytical technology.

Recent recalls have contributed to the pharmaceutical industry?s heightened awareness of glass delamination (i.e., the formation of glass flakes in a vial), which could affect drug quality and patient safety. To confront this growing problem effectively, drugmakers must understand its causes.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.

The authors summarize a recent FDA–PQRI workshop on process drift.
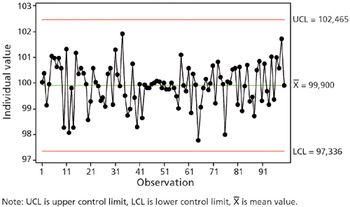
Current GMPs demand full understandng of out-of-control concepts. This article contains bonus material.

New product reviews for October 2011 focusing on analytical instrumentation.

The authors summarize the Matrixx Initiatives, Inc. v. Siracusano case's implications for industry.